Difference between revisions of "Timeline of malaria vaccine"
| (18 intermediate revisions by 2 users not shown) | |||
| Line 21: | Line 21: | ||
|- | |- | ||
| 1942 || Immunology development || Studies of inactivated sporozoite immunization show apparently beneficial effect of combining induction of cellular and humoral [[wikipedia:immune response|immune responses]] against malaria of domestic [[wikipedia:fowl|fowl]].<ref name="Vaccines against malaria"/> || | | 1942 || Immunology development || Studies of inactivated sporozoite immunization show apparently beneficial effect of combining induction of cellular and humoral [[wikipedia:immune response|immune responses]] against malaria of domestic [[wikipedia:fowl|fowl]].<ref name="Vaccines against malaria"/> || | ||
| + | |- | ||
| + | | 1950s || Immunology development || Research finds that immunization of [[wikipedia:chicken|chickens]] with a mix of asexual and sexual stages of ''[[wikipedia:plasmodium gallinaceum|plasmodium gallinaceum]]'' blocks parasite infectivity.<ref name="Transmission blocking malaria vaccines: Assays and candidates in clinical development">{{cite journal|title=Transmission blocking malaria vaccines: Assays and candidates in clinical development|doi=10.1016/j.vaccine.2015.08.073|url=http://www.sciencedirect.com/science/article/pii/S0264410X1501213X|accessdate=25 April 2017}}</ref> || | ||
|- | |- | ||
| 1967 || Immunology development || Research on [[wikipedia:avian malaria|avian malaria]] shows that killed sporozoites as well as sporozoites inactivated with [[wikipedia:ultraviolet light|ultraviolet light]] can produce a partial [[wikipedia:immunity|immunity]] after injection into [[wikipedia:birds|birds]].<ref>{{cite web|title=Protective Immunity produced by the Injection of X-irradiated Sporozoites of Plasmodium berghei|url=http://www.nature.com/nature/journal/v216/n5111/abs/216160a0.html|website=nature.com|accessdate=18 April 2017}}</ref><ref name="Malaria Vaccine Design: Immunological Considerations">{{cite web|title=Malaria Vaccine Design: Immunological Considerations|url=http://www.sciencedirect.com/science/article/pii/S1074761310003651|website=sciencedirect.com|accessdate=18 April 2017}}</ref> During the 1960s, the path to malaria vaccine development consolidates with immunization studies of [[wikipedia:mouse|mice]] with irradiated sporozoites, and posterior analyses of the mechanisms of immunity in this model.<ref name="Vaccines against malaria">{{cite web|title=Vaccines against malaria|url=http://rstb.royalsocietypublishing.org/content/366/1579/2806|website=royalsocietypublishing.org|accessdate=18 April 2017}}</ref> || | | 1967 || Immunology development || Research on [[wikipedia:avian malaria|avian malaria]] shows that killed sporozoites as well as sporozoites inactivated with [[wikipedia:ultraviolet light|ultraviolet light]] can produce a partial [[wikipedia:immunity|immunity]] after injection into [[wikipedia:birds|birds]].<ref>{{cite web|title=Protective Immunity produced by the Injection of X-irradiated Sporozoites of Plasmodium berghei|url=http://www.nature.com/nature/journal/v216/n5111/abs/216160a0.html|website=nature.com|accessdate=18 April 2017}}</ref><ref name="Malaria Vaccine Design: Immunological Considerations">{{cite web|title=Malaria Vaccine Design: Immunological Considerations|url=http://www.sciencedirect.com/science/article/pii/S1074761310003651|website=sciencedirect.com|accessdate=18 April 2017}}</ref> During the 1960s, the path to malaria vaccine development consolidates with immunization studies of [[wikipedia:mouse|mice]] with irradiated sporozoites, and posterior analyses of the mechanisms of immunity in this model.<ref name="Vaccines against malaria">{{cite web|title=Vaccines against malaria|url=http://rstb.royalsocietypublishing.org/content/366/1579/2806|website=royalsocietypublishing.org|accessdate=18 April 2017}}</ref> || | ||
| Line 32: | Line 34: | ||
| 1985 || Immunology development || Researchers find that immunization with processed fragments of called Merozoite surface protein-1 MSP-1 (one of the most studied of all malaria [[wikipedia:protein|proteins]]) induce protective immune responses.<ref name="Malaria vaccines 1985–2005: a full circle?">{{cite web|title=Malaria vaccines 1985–2005: a full circle?|url=http://www.columbia.edu/itc/hs/medical/pathophys/id/2005/Larussa-TargettMalariaVacs.pdf|website=columbia.edu|accessdate=22 April 2017}}</ref> || | | 1985 || Immunology development || Researchers find that immunization with processed fragments of called Merozoite surface protein-1 MSP-1 (one of the most studied of all malaria [[wikipedia:protein|proteins]]) induce protective immune responses.<ref name="Malaria vaccines 1985–2005: a full circle?">{{cite web|title=Malaria vaccines 1985–2005: a full circle?|url=http://www.columbia.edu/itc/hs/medical/pathophys/id/2005/Larussa-TargettMalariaVacs.pdf|website=columbia.edu|accessdate=22 April 2017}}</ref> || | ||
|- | |- | ||
| − | | 1986-1987 || Immunology development || Team lead by Colombian Professor [[wikipedia:Manuel Elkin Patarroyo|Manuel Elkin Patarroyo]] inoculates hundreds of [[wikipedia:aotus monkey|aotus monkeys]] with experimental synthetic proteins designed to imitate bite of malaria proteins. By 1987 Patarroyo | + | | 1986-1987 || Immunology development || Team lead by Colombian Professor [[wikipedia:Manuel Elkin Patarroyo|Manuel Elkin Patarroyo]] inoculates hundreds of [[wikipedia:aotus monkey|aotus monkeys]] with experimental synthetic proteins designed to imitate bite of malaria proteins. By 1987 Patarroyo reports in [[wikipedia:Nature Magazine|Nature Magazine]] that three particular synthetic proteins protect a significant proportion of monkeys. However, a carrier molecule used to present the peptides to the monkeys’ immune systems appears to damage the animals’ livers.<ref name="One Colombian's Quest For A Malaria Vaccine">{{cite web|title=One Colombian's Quest For A Malaria Vaccine|url=http://www.the-scientist.com/?articles.view/articleNo/10052/title/One-Colombian-s-Quest-For-A-Malaria-Vaccine/|website=the-scientist.com|accessdate=22 April 2017}}</ref> || [[wikipedia:Colombia|Colombia]] |
|- | |- | ||
| 1987 || Vaccine development || Researchers working at [[wikipedia:GlaxoSmithKline|GlaxoSmithKline]]’s (GSK) laboratories, develop malaria vaccine candidate [[wikipedia:RTS,S|RTS,S]] (Mosquirix TM).<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/> It is the first vaccine created by combining the malaria CS protein and [[wikipedia:hepatitis B|hepatitis B]] surface antigen.<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/> || [[wikipedia:United Kingdom|United Kingdom]] | | 1987 || Vaccine development || Researchers working at [[wikipedia:GlaxoSmithKline|GlaxoSmithKline]]’s (GSK) laboratories, develop malaria vaccine candidate [[wikipedia:RTS,S|RTS,S]] (Mosquirix TM).<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/> It is the first vaccine created by combining the malaria CS protein and [[wikipedia:hepatitis B|hepatitis B]] surface antigen.<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/> || [[wikipedia:United Kingdom|United Kingdom]] | ||
| Line 44: | Line 46: | ||
| 1998 || Vaccine development (trial) || First [[wikipedia:RTS,S|RTS,S]] vaccine trials in [[wikipedia:Africa|Africa]] are conducted in Gambia.<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/> || [[wikipedia:Gambia|Gambia]] | | 1998 || Vaccine development (trial) || First [[wikipedia:RTS,S|RTS,S]] vaccine trials in [[wikipedia:Africa|Africa]] are conducted in Gambia.<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/> || [[wikipedia:Gambia|Gambia]] | ||
|- | |- | ||
| − | | 1999 || Program launch || The [[wikipedia:PATH (global health organization)#Malaria|PATH Malaria Vaccine Initiative (MVI)]] is established, with aims at accelerating the development of malaria vaccines and catalyzing timely access in endemic countries. <ref name="Mosquirix™"/><ref>{{cite web|title=Malaria Vaccine Initiative|url=http://www.inyvax.eu/mvi|website=inyvax.eu|accessdate=18 April 2017}}</ref> || | + | | 1999 || Program launch || The [[wikipedia:PATH (global health organization)#Malaria|PATH Malaria Vaccine Initiative (MVI)]] is established, with aims at accelerating the development of malaria vaccines and catalyzing timely access in endemic countries. <ref name="Mosquirix™"/><ref>{{cite web|title=Malaria Vaccine Initiative|url=http://www.inyvax.eu/mvi|website=inyvax.eu|accessdate=18 April 2017}}</ref> The program launches with a $50 million grant from the [[wikipedia:Bill & Melinda Gates Foundation|Bill & Melinda Gates Foundation]].<ref>{{cite web|title=PATH Malaria Vaccine Initiative (MVI): Accelerating Malaria Vaccine Development|url=https://www.devex.com/impact/partnerships/path-malaria-vaccine-initiative-mvi-249|website=devex.com|accessdate=25 April 2017}}</ref> || |
|- | |- | ||
| 2001 || Organization || The GSK/MVI partnership ([[wikipedia:GlaxoSmithKline|GlaxoSmithKline]] and [[wikipedia:PATH (global health organization)#Malaria|PATH Malaria Vaccine Initiative (MVI)]]) initiates, with grants from the [[wikipedia:Bill & Melinda Gates Foundation|Bill & Melinda Gates Foundation]] to PATH, with aims at developing [[wikipedia:RTS,S|RTS,S]] vaccine for infants and young children living in malaria-endemic regions in [[wikipedia:Sub-Saharan Africa|Sub-Saharan Africa]]."<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/>|| | | 2001 || Organization || The GSK/MVI partnership ([[wikipedia:GlaxoSmithKline|GlaxoSmithKline]] and [[wikipedia:PATH (global health organization)#Malaria|PATH Malaria Vaccine Initiative (MVI)]]) initiates, with grants from the [[wikipedia:Bill & Melinda Gates Foundation|Bill & Melinda Gates Foundation]] to PATH, with aims at developing [[wikipedia:RTS,S|RTS,S]] vaccine for infants and young children living in malaria-endemic regions in [[wikipedia:Sub-Saharan Africa|Sub-Saharan Africa]]."<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )"/>|| | ||
|- | |- | ||
| − | | 2003 || Vaccine development (trial) || RTS,S/AS02A malaria vaccine candidate phase IIb clinical trial is conducted in children from 1 to 4 years of age in Mozambique. The vaccine shows to be safe, immunogenic and efficacious, reducing plasmodium falciparum clinical malaria cases by 30% and episodes of severe disease by up to 58%. In addition, protection would appear to last for a 18 month follow‐up period.<ref>{{cite journal|title=Towards an effective malaria vaccine|doi=10.1136/adc.2005.092551|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2066178/|accessdate=25 April 2017|pmc=2066178}}</ref> || [[wikipedia:Mozambique|Mozambique]] | + | | 2003 || Vaccine development (trial) || RTS,S/AS02A malaria vaccine candidate phase IIb clinical trial is conducted in children from 1 to 4 years of age in Mozambique. The vaccine shows to be safe, immunogenic and efficacious, reducing ''[[wikipedia:plasmodium falciparum|plasmodium falciparum]]'' clinical malaria cases by 30% and episodes of severe disease by up to 58%. In addition, protection would appear to last for a 18 month follow‐up period.<ref>{{cite journal|title=Towards an effective malaria vaccine|doi=10.1136/adc.2005.092551|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2066178/|accessdate=25 April 2017|pmc=2066178}}</ref> || [[wikipedia:Mozambique|Mozambique]] |
|- | |- | ||
| 2006 || Program launch || The [[wikipedia:Malaria Vaccine Technology Roadmap|Malaria Vaccine Technology Roadmap]] launches.<ref name="Immunization, Vaccines and Biologicals">{{cite web|title=Immunization, Vaccines and Biologicals|url=http://www.who.int/immunization/topics/en/|website=who.int|accessdate=17 April 2017}}</ref><ref>{{cite journal|title=Malaria vaccine technology roadmap|doi=10.1016/S0140-6736(13)62238-2|url=http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)62238-2/fulltext|accessdate=18 April 2017}}</ref> || | | 2006 || Program launch || The [[wikipedia:Malaria Vaccine Technology Roadmap|Malaria Vaccine Technology Roadmap]] launches.<ref name="Immunization, Vaccines and Biologicals">{{cite web|title=Immunization, Vaccines and Biologicals|url=http://www.who.int/immunization/topics/en/|website=who.int|accessdate=17 April 2017}}</ref><ref>{{cite journal|title=Malaria vaccine technology roadmap|doi=10.1016/S0140-6736(13)62238-2|url=http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)62238-2/fulltext|accessdate=18 April 2017}}</ref> || | ||
| Line 64: | Line 66: | ||
| 2015 || Policy || The [[wikipedia:Committee for Medicinal Products for Human Use|Committee for Medicinal Products for Human Use]] (CHMP) of the [[wikipedia:European Medicines Agency|European Medicines Agency]] (EMA) announces having adopted a positive scientific opinion for [[wikipedia:GlaxoSmithKline|GSK]]’s malaria candidate vaccine [[wikipedia:RTS,S|RTS,S]] (Mosquirix TM), in children aged 6 weeks to 17 months.<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )">{{cite web|title=Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )|url=http://www.malariavaccine.org/files/MVI-GSK-RTSSfactsheetFINAL-web.pdf|accessdate=17 April 2017}}</ref><ref name="Questions and answers on RTS,S/ASO1 malaria vaccine"/> || | | 2015 || Policy || The [[wikipedia:Committee for Medicinal Products for Human Use|Committee for Medicinal Products for Human Use]] (CHMP) of the [[wikipedia:European Medicines Agency|European Medicines Agency]] (EMA) announces having adopted a positive scientific opinion for [[wikipedia:GlaxoSmithKline|GSK]]’s malaria candidate vaccine [[wikipedia:RTS,S|RTS,S]] (Mosquirix TM), in children aged 6 weeks to 17 months.<ref name="Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )">{{cite web|title=Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )|url=http://www.malariavaccine.org/files/MVI-GSK-RTSSfactsheetFINAL-web.pdf|accessdate=17 April 2017}}</ref><ref name="Questions and answers on RTS,S/ASO1 malaria vaccine"/> || | ||
|- | |- | ||
| − | | 2016 || Policy || Following the opinion of the [[wikipedia:Committee for Medicinal Products for Human Use|CHMP]], The [[wikipedia:World Health | + | | 2016 || Medical development || Biotechnology corporation [[wikipedia:Sanaria|Sanaria]] reports experimental malaria vaccine tested in varying doses providing 55 percent protection for one year to a few volunteers. The vaccine, made by the company, contains thousands of live malaria parasites weakened by [[wikipedia:radiation|radiation]]; the parasites would create an immune response but do not persist in the body for long.<ref>{{cite web|title=A Malaria Vaccine Has Some Success in Testing|url=https://www.nytimes.com/2016/05/10/health/a-malaria-vaccine-has-some-success-in-testing.html?_r=0|website=nytimes.com|accessdate=16 June 2017}}</ref> || United States |
| + | |- | ||
| + | | 2016 || Policy || Following the opinion of the [[wikipedia:Committee for Medicinal Products for Human Use|CHMP]], The [[wikipedia:World Health Organization|World Health Organization]] recommends that the pilot implementations use the 4-dose schedule of the RTS,S/AS01 vaccine in 3–5 distinct epidemiological settings in sub-Saharan Africa, at subnational level, covering moderate-to-high transmission settings, with three doses administered to children between 5 and 9 months of age, followed by a fourth dose 15–18 months later.<ref name="Mosquirix™"/><ref name="Questions and answers on RTS,S/ASO1 malaria vaccine"/><ref name="Questions and answers on RTS,S/ASO1 malaria vaccine">{{cite web|title=Questions and answers on RTS,S/ASO1 malaria vaccine|url=http://www.who.int/immunization/research/development/malaria_vaccine_qa/en/|website=who.int|accessdate=17 April 2017}}</ref> | ||
| + | |- | ||
| + | | 2017 || Report || The World Health Organization Regional Office for Africa (WHO/AFRO) announces on April 24, that Ghana, Kenya, and Malawi will partner with WHO in the Malaria Vaccine Implementation Programme (MVIP) that will make the [[wikipedia:RTS,S|RTS,S]] vaccine available in selected areas of the three countries.<ref>{{cite web|title=RTS,S|url=http://www.malariavaccine.org/malaria-and-vaccines/first-generation-vaccine/rtss|website=malariavaccine.org|accessdate=25 April 2017}}</ref> || [[wikipedia:Ghana|Ghana]], [[wikipedia:Kenya|Kenya]], [[wikipedia:Malawi|Malawi]] | ||
| + | |- | ||
| + | | 2017 || Medical development || Research team at [[wikipedia:University of Tübingen|University of Tübingen]] in collaboration with biotech company [[wikipedia:Sanaria|Sanaria]] demonstrate 100% effectiveness in clinical trial of new malaria vaccine (Sanaria PfSPZ-CVac), when assessed at 10 weeks after last dose of the vaccine.<ref>{{cite web|title=New malaria vaccine effective in clinical trial|url=https://www.sciencedaily.com/releases/2017/02/170215131606.htm|website=sciencedaily.com|accessdate=16 June 2017}}</ref> || [[wikipedia:Germany|Germany]] | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | == Visual data == | ||
| + | |||
| + | === Google Trends === | ||
| + | |||
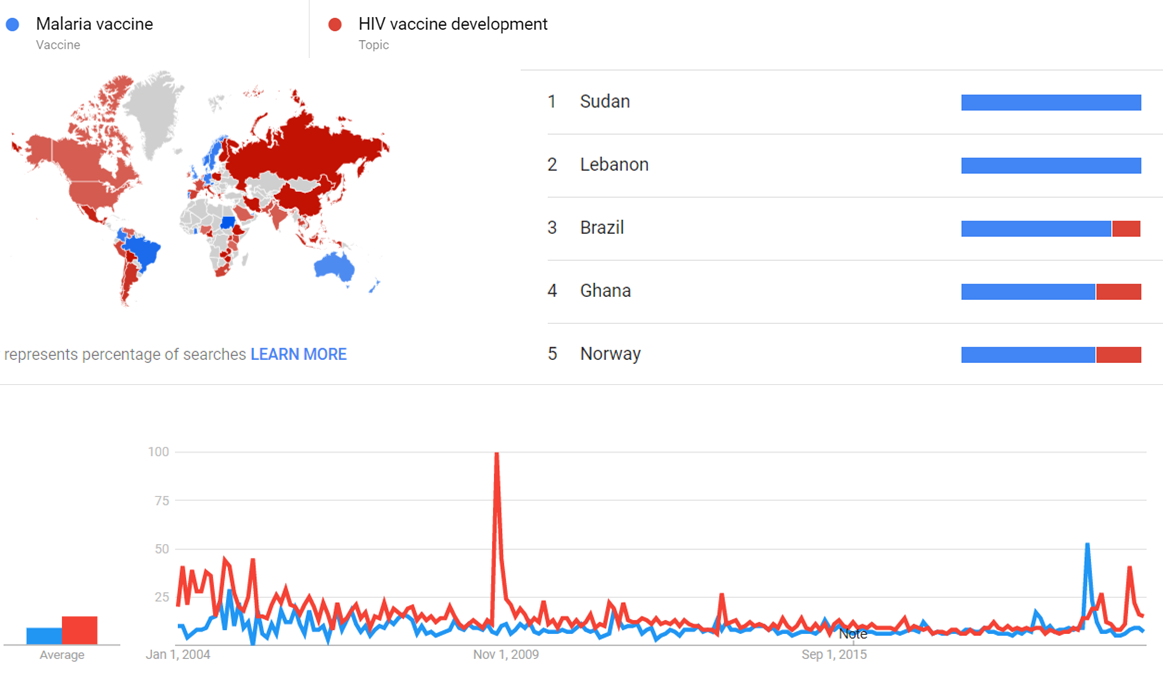
| + | The comparative chart below shows {{w|Google Trends}} data for Malaria vaccine (Vaccine) and HIV vaccine development (Topic), from January 2004 to March 2020, when the screenshot was taken. Interest is also ranked by country and displayed on world map.<ref>{{cite web |title=Malaria vaccine and HIV vaccine development |url=https://trends.google.com/trends/explore?date=all&q=%2Fm%2F02q78jt,%2Fm%2F012vyq |website=Google Trends |access-date=30 March 2021}}</ref> | ||
| + | |||
| + | [[File:Malaria vaccine and HIV vaccine development gt.png|thumb|center|600px]] | ||
| + | |||
| + | === Google Ngram Viewer === | ||
| + | |||
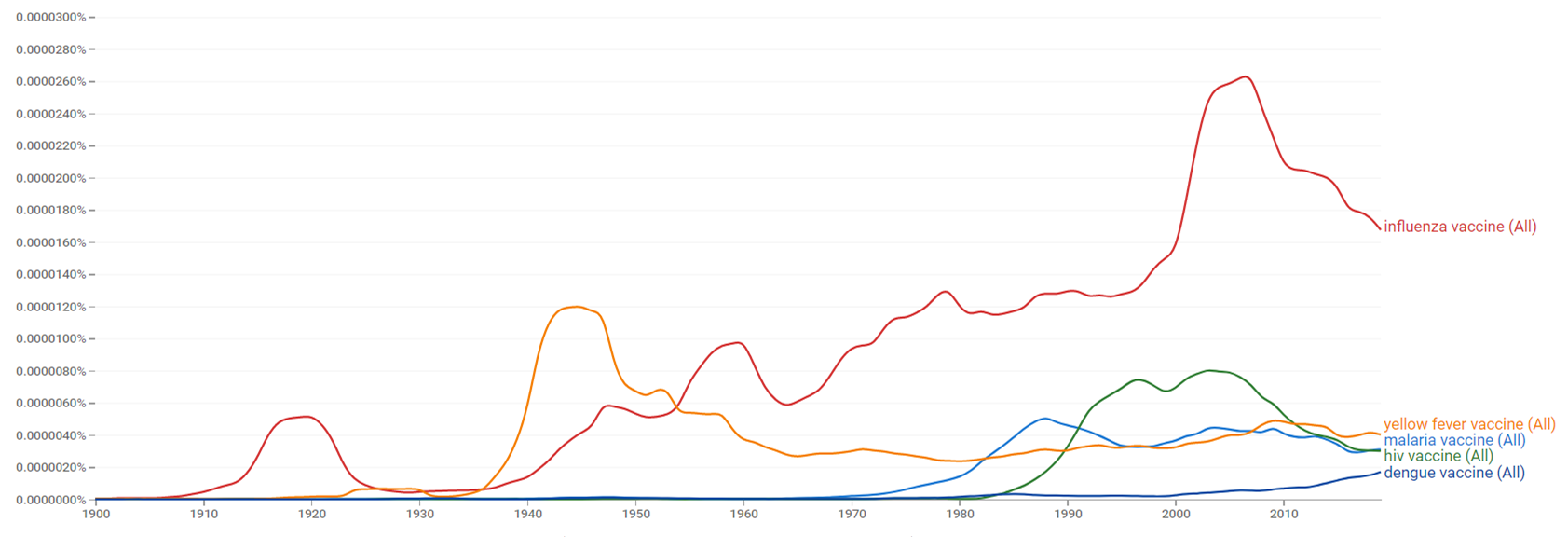
| + | The comparative chart below shows {{w|Google Ngram Viewer}} data for malaria vaccine, influenza vaccine, hiv vaccine, yellow fever vaccine and dengue vaccine, from 1900 to 2019.<ref>{{cite web |title=malaria vaccine, influenza vaccine, hiv vaccine, yellow fever vaccine and dengue vaccine |url=https://books.google.com/ngrams/graph?content=malaria+vaccine%2Cinfluenza+vaccine%2Chiv+vaccine%2Cyellow+fever+vaccine%2Cdengue+vaccine&year_start=1900&year_end=2019&case_insensitive=on&corpus=26&smoothing=3&direct_url=t4%3B%2Cmalaria%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Bmalaria%20vaccine%3B%2Cc0%3B%3BMalaria%20Vaccine%3B%2Cc0%3B%3BMalaria%20vaccine%3B%2Cc0%3B%3BMALARIA%20VACCINE%3B%2Cc0%3B.t4%3B%2Cinfluenza%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Binfluenza%20vaccine%3B%2Cc0%3B%3BInfluenza%20Vaccine%3B%2Cc0%3B%3BInfluenza%20vaccine%3B%2Cc0%3B%3BINFLUENZA%20VACCINE%3B%2Cc0%3B.t4%3B%2Chiv%20vaccine%3B%2Cc0%3B%2Cs0%3B%3BHIV%20vaccine%3B%2Cc0%3B%3BHIV%20Vaccine%3B%2Cc0%3B%3BHIV%20VACCINE%3B%2Cc0%3B.t4%3B%2Cyellow%20fever%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Byellow%20fever%20vaccine%3B%2Cc0%3B%3BYellow%20fever%20vaccine%3B%2Cc0%3B%3BYellow%20Fever%20Vaccine%3B%2Cc0%3B%3BYELLOW%20FEVER%20VACCINE%3B%2Cc0%3B%3BYellow%20Fever%20vaccine%3B%2Cc0%3B.t4%3B%2Cdengue%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Bdengue%20vaccine%3B%2Cc0%3B%3BDengue%20vaccine%3B%2Cc0%3B%3BDengue%20Vaccine%3B%2Cc0#t4%3B%2Cmalaria%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Bmalaria%20vaccine%3B%2Cc0%3B%3BMalaria%20Vaccine%3B%2Cc0%3B%3BMalaria%20vaccine%3B%2Cc0%3B%3BMALARIA%20VACCINE%3B%2Cc0%3B.t4%3B%2Cinfluenza%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Binfluenza%20vaccine%3B%2Cc0%3B%3BInfluenza%20Vaccine%3B%2Cc0%3B%3BInfluenza%20vaccine%3B%2Cc0%3B%3BINFLUENZA%20VACCINE%3B%2Cc0%3B.t4%3B%2Chiv%20vaccine%3B%2Cc0%3B%2Cs0%3B%3BHIV%20vaccine%3B%2Cc0%3B%3BHIV%20Vaccine%3B%2Cc0%3B%3BHIV%20VACCINE%3B%2Cc0%3B.t4%3B%2Cyellow%20fever%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Byellow%20fever%20vaccine%3B%2Cc0%3B%3BYellow%20fever%20vaccine%3B%2Cc0%3B%3BYellow%20Fever%20Vaccine%3B%2Cc0%3B%3BYELLOW%20FEVER%20VACCINE%3B%2Cc0%3B%3BYellow%20Fever%20vaccine%3B%2Cc0%3B.t4%3B%2Cdengue%20vaccine%3B%2Cc0%3B%2Cs0%3B%3Bdengue%20vaccine%3B%2Cc0%3B%3BDengue%20vaccine%3B%2Cc0%3B%3BDengue%20Vaccine%3B%2Cc0 |website=books.google.com |access-date=30 March 2021 |language=en}}</ref> | ||
| + | |||
| + | [[File:Malaria vaccine, influenza vaccine, hiv vaccine, yellow fever vaccine and dengue vaccine ngram.png|thumb|center|700px]] | ||
| + | |||
| + | === Wikipedia Views === | ||
| + | |||
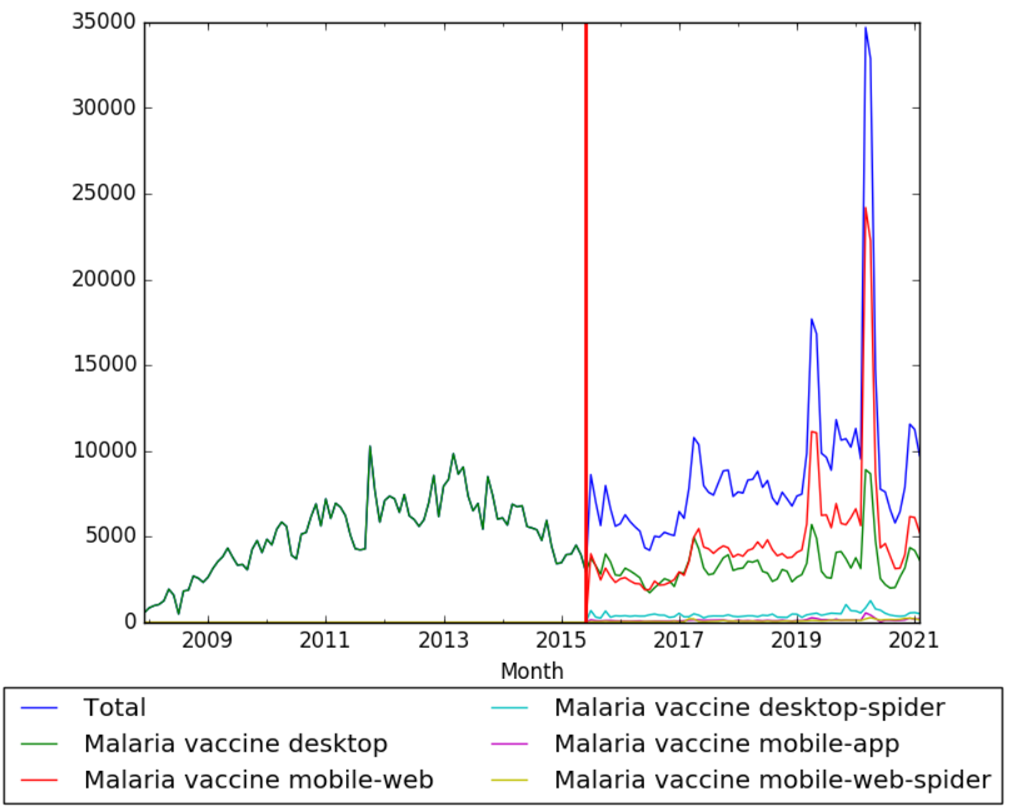
| + | The chart below shows pageviews of the English Wikipedia article {{w|Malaria vaccine}}, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to February 2021.<ref>{{cite web |title=Malaria vaccine |url=https://wikipediaviews.org/displayviewsformultiplemonths.php?page=Malaria+vaccine&allmonths=allmonths&language=en&drilldown=all |website=wikipediaviews.org |access-date=17 March 2021}}</ref> | ||
| + | |||
| + | [[File:Malaria vaccine wv.png|thumb|center|400px]] | ||
| + | |||
| + | ==Meta information on the timeline== | ||
| + | |||
| + | ===How the timeline was built=== | ||
| + | |||
| + | The initial version of the timeline was written by [[User:FIXME|FIXME]]. | ||
| + | |||
| + | {{funding info}} is available. | ||
| + | |||
| + | ===Feedback and comments=== | ||
| + | |||
| + | Feedback for the timeline can be provided at the following places: | ||
| + | |||
| + | * FIXME | ||
| + | |||
| + | ===What the timeline is still missing=== | ||
| + | |||
| + | * [https://www.frontiersin.org/articles/10.3389/fimmu.2020.00204/full] | ||
| + | |||
| + | ===Timeline update strategy=== | ||
| + | |||
| + | === Pingbacks === | ||
| + | |||
| + | * [https://books.google.com.ar/books?id=vcdUEAAAQBAJ&pg=PA518&lpg=PA518&dq=timelines.issarice&source=bl&ots=QaZM_D_nxk&sig=ACfU3U2plxF7RA4Hylip_sePrDCy40F23Q&hl=en&sa=X&ved=2ahUKEwjx67Ct1--CAxXER7gEHVKkAUk4ChDoAXoECAIQAw#v=onepage&q=timelines.issarice&f=false ''FAQ on Vaccines and Immunization Practices'', by Vipin Vashishtha and Ajay Kalra] Reference N°6 at page 518 | ||
==See also== | ==See also== | ||
| Line 72: | Line 124: | ||
* [[Timeline of malaria]] | * [[Timeline of malaria]] | ||
* [[Timeline of mosquito net distribution]] | * [[Timeline of mosquito net distribution]] | ||
| + | * [[Speculative timeline of future malaria events]] | ||
| + | |||
| + | ==External links== | ||
| + | |||
| + | ==References== | ||
| − | + | {{Reflist|30em}} | |
Latest revision as of 20:26, 1 December 2023
This is a timeline of malaria vaccine, attempting to describe its development. Efforts to develop effective vaccines span for more than six decades.[1]
Contents
Big picture
| Year/period | Key development |
|---|---|
| 1940s-1984 | Malaria immunization studies are conducted with aims at developing vaccines.[1] |
| 1984 onward | RTS,S, the first malaria vaccine candidate is developed.[2] |
| 1995 onward | RTS,S malaria vaccine candidate trials start to be conducted, mostly in Africa.[2] |
| Present time | No effective malaria vaccine exists as of yet.[3] |
Timeline
| Year/period | Type of event | Event | Location |
|---|---|---|---|
| 1942 | Immunology development | Studies of inactivated sporozoite immunization show apparently beneficial effect of combining induction of cellular and humoral immune responses against malaria of domestic fowl.[1] | |
| 1950s | Immunology development | Research finds that immunization of chickens with a mix of asexual and sexual stages of plasmodium gallinaceum blocks parasite infectivity.[4] | |
| 1967 | Immunology development | Research on avian malaria shows that killed sporozoites as well as sporozoites inactivated with ultraviolet light can produce a partial immunity after injection into birds.[5][6] During the 1960s, the path to malaria vaccine development consolidates with immunization studies of mice with irradiated sporozoites, and posterior analyses of the mechanisms of immunity in this model.[1] | |
| 1970 | Immunology development | Research reveals immune cross protection against malaria in rodent systems.[7] | |
| 1983 | Publication | The first publications for cloning malaria antigens appear.[6] | |
| 1984 | Program launch | The Walter Reed Army Institute of Research (WRAIR) and British pharmaceutical company GlaxoSmithKline (GSK) initiate collaboration to produce a malaria vaccine using GSK’s recombinant escherichia coli expression systems.[2][8] | |
| 1985 | Immunology development | Researchers find that immunization with processed fragments of called Merozoite surface protein-1 MSP-1 (one of the most studied of all malaria proteins) induce protective immune responses.[9] | |
| 1986-1987 | Immunology development | Team lead by Colombian Professor Manuel Elkin Patarroyo inoculates hundreds of aotus monkeys with experimental synthetic proteins designed to imitate bite of malaria proteins. By 1987 Patarroyo reports in Nature Magazine that three particular synthetic proteins protect a significant proportion of monkeys. However, a carrier molecule used to present the peptides to the monkeys’ immune systems appears to damage the animals’ livers.[10] | Colombia |
| 1987 | Vaccine development | Researchers working at GlaxoSmithKline’s (GSK) laboratories, develop malaria vaccine candidate RTS,S (Mosquirix TM).[2] It is the first vaccine created by combining the malaria CS protein and hepatitis B surface antigen.[2] | United Kingdom |
| 1993 | Vaccine development | Study on multi-stage, multi-component malaria vaccine cocktail featuring pre-erythrocytic, blood, and sexual stage antigens gives favourable results, however, further clinical development of this approach would not be reported.[11] | |
| 1995 | Vaccine development (trial) | First RTS,S vaccine clinical tests in humans are conducted in adults in the United States.[2] | United States |
| 1997 | Vaccine development (trial) | RTS,S vaccine Key proof-of-concept (PoC) study shows 100% protection in 6 of 7 volunteers in challenge trial.[2] | |
| 1998 | Vaccine development (trial) | First RTS,S vaccine trials in Africa are conducted in Gambia.[2] | Gambia |
| 1999 | Program launch | The PATH Malaria Vaccine Initiative (MVI) is established, with aims at accelerating the development of malaria vaccines and catalyzing timely access in endemic countries. [12][13] The program launches with a $50 million grant from the Bill & Melinda Gates Foundation.[14] | |
| 2001 | Organization | The GSK/MVI partnership (GlaxoSmithKline and PATH Malaria Vaccine Initiative (MVI)) initiates, with grants from the Bill & Melinda Gates Foundation to PATH, with aims at developing RTS,S vaccine for infants and young children living in malaria-endemic regions in Sub-Saharan Africa."[2] | |
| 2003 | Vaccine development (trial) | RTS,S/AS02A malaria vaccine candidate phase IIb clinical trial is conducted in children from 1 to 4 years of age in Mozambique. The vaccine shows to be safe, immunogenic and efficacious, reducing plasmodium falciparum clinical malaria cases by 30% and episodes of severe disease by up to 58%. In addition, protection would appear to last for a 18 month follow‐up period.[15] | Mozambique |
| 2006 | Program launch | The Malaria Vaccine Technology Roadmap launches.[16][17] | |
| 2007 | Publication | Phase II results in African children and infants are published in The Lancet and The New England Journal of Medicine.[2] | |
| 2009-2014 | Vaccine development (trial) | RTS,S vaccine phase III study is launched in Kisumu, Kenya, in July, under the auspices of the Kenya Medical Research Institute (KEMRI)/CDC Research and Public Health Collaboration.[2][18] The trial would end in 2014, involving 15,459 infants and young children at 11 sites in seven African countries, being the largest malaria vaccine trial in Africa to date.[12][19] | Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and Tanzania |
| 2011 | Vaccine development (trial) | RTS,S vaccine Phase III trials results, released in October, show that in children aged 5-17 months, vaccination with RTS,S reduce the risk of clinical malaria and severe malaria by 56% and 47%, respectively. However, further results released show the vaccine less effective in infants aged 6-12 weeks at first vaccination.[20] | Africa |
| 2013 | Update | The Malaria Vaccine Technology Roadmap (launched in 2006) is updated in November. The update represents a blueprint for second generation malaria vaccine development, including a new Vision, two new Strategic Goals and 13 priority activities.[16] | |
| 2014 | Vaccine development (trial) | Initial phase III result at 18 months of RTS,S trial introduction shows the vaccine efficacy of 46% in children and 27% among young infants against the clinical malaria.[21] | |
| 2015 | Policy | The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) announces having adopted a positive scientific opinion for GSK’s malaria candidate vaccine RTS,S (Mosquirix TM), in children aged 6 weeks to 17 months.[2][22] | |
| 2016 | Medical development | Biotechnology corporation Sanaria reports experimental malaria vaccine tested in varying doses providing 55 percent protection for one year to a few volunteers. The vaccine, made by the company, contains thousands of live malaria parasites weakened by radiation; the parasites would create an immune response but do not persist in the body for long.[23] | United States |
| 2016 | Policy | Following the opinion of the CHMP, The World Health Organization recommends that the pilot implementations use the 4-dose schedule of the RTS,S/AS01 vaccine in 3–5 distinct epidemiological settings in sub-Saharan Africa, at subnational level, covering moderate-to-high transmission settings, with three doses administered to children between 5 and 9 months of age, followed by a fourth dose 15–18 months later.[12][22][22] | |
| 2017 | Report | The World Health Organization Regional Office for Africa (WHO/AFRO) announces on April 24, that Ghana, Kenya, and Malawi will partner with WHO in the Malaria Vaccine Implementation Programme (MVIP) that will make the RTS,S vaccine available in selected areas of the three countries.[24] | Ghana, Kenya, Malawi |
| 2017 | Medical development | Research team at University of Tübingen in collaboration with biotech company Sanaria demonstrate 100% effectiveness in clinical trial of new malaria vaccine (Sanaria PfSPZ-CVac), when assessed at 10 weeks after last dose of the vaccine.[25] | Germany |
Visual data
Google Trends
The comparative chart below shows Google Trends data for Malaria vaccine (Vaccine) and HIV vaccine development (Topic), from January 2004 to March 2020, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[26]
Google Ngram Viewer
The comparative chart below shows Google Ngram Viewer data for malaria vaccine, influenza vaccine, hiv vaccine, yellow fever vaccine and dengue vaccine, from 1900 to 2019.[27]
Wikipedia Views
The chart below shows pageviews of the English Wikipedia article Malaria vaccine, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to February 2021.[28]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by FIXME.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
Timeline update strategy
Pingbacks
- FAQ on Vaccines and Immunization Practices, by Vipin Vashishtha and Ajay Kalra Reference N°6 at page 518
See also
- Timeline of malaria
- Timeline of mosquito net distribution
- Speculative timeline of future malaria events
External links
References
- ↑ 1.0 1.1 1.2 1.3 "Vaccines against malaria". royalsocietypublishing.org. Retrieved 18 April 2017.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "Fact s heet: T he RTS,S malaria vaccine candidate (Mosquirix TM )" (PDF). Retrieved 17 April 2017.
- ↑ "Current Obstacles to Development THE SCIENCE". nap.edu. Retrieved 24 April 2017.
- ↑ "Transmission blocking malaria vaccines: Assays and candidates in clinical development". doi:10.1016/j.vaccine.2015.08.073. Retrieved 25 April 2017.
- ↑ "Protective Immunity produced by the Injection of X-irradiated Sporozoites of Plasmodium berghei". nature.com. Retrieved 18 April 2017.
- ↑ 6.0 6.1 "Malaria Vaccine Design: Immunological Considerations". sciencedirect.com. Retrieved 18 April 2017.
- ↑ Peters, Wallace. Antimalarial Drugs I: Biological Background, Experimental Methods, and Drug Resistance. Retrieved 19 April 2017.
- ↑ Ripley Ballou, W.; Cahill, Conor P. "Two Decades of Commitment to Malaria Vaccine Development: Glaxosmithkline Biologicals". Retrieved 19 April 2017.
- ↑ "Malaria vaccines 1985–2005: a full circle?" (PDF). columbia.edu. Retrieved 22 April 2017.
- ↑ "One Colombian's Quest For A Malaria Vaccine". the-scientist.com. Retrieved 22 April 2017.
- ↑ "Analysis of a Multi-component Multi-stage Malaria Vaccine Candidate—Tackling the Cocktail Challenge". plos.org. doi:10.1371/journal.pone.0131456. Retrieved 25 April 2017.
- ↑ 12.0 12.1 12.2 "Fact sheet: R TS,S malaria vaccine candidate (Mosquirix™)" (PDF). malariavaccine.org. Retrieved 17 April 2017.
- ↑ "Malaria Vaccine Initiative". inyvax.eu. Retrieved 18 April 2017.
- ↑ "PATH Malaria Vaccine Initiative (MVI): Accelerating Malaria Vaccine Development". devex.com. Retrieved 25 April 2017.
- ↑ "Towards an effective malaria vaccine". PMC 2066178
 . doi:10.1136/adc.2005.092551. Retrieved 25 April 2017.
. doi:10.1136/adc.2005.092551. Retrieved 25 April 2017.
- ↑ 16.0 16.1 "Immunization, Vaccines and Biologicals". who.int. Retrieved 17 April 2017.
- ↑ "Malaria vaccine technology roadmap". doi:10.1016/S0140-6736(13)62238-2. Retrieved 18 April 2017.
- ↑ "Malaria Vaccine". cdc.gov. Retrieved 17 April 2017.
- ↑ "Advances and challenges in malaria vaccine development". PMC 2994342
 . doi:10.1172/JCI44423. Retrieved 17 April 2017.
. doi:10.1172/JCI44423. Retrieved 17 April 2017.
- ↑ "Malaria and Malaria Vaccine Candidates". historyofvaccines.org. Retrieved 17 April 2017.
- ↑ "Malaria Vaccine Development: Recent Advances alongside the Barriers". omicsonline.org. Retrieved 18 April 2017.
- ↑ 22.0 22.1 22.2 "Questions and answers on RTS,S/ASO1 malaria vaccine". who.int. Retrieved 17 April 2017.
- ↑ "A Malaria Vaccine Has Some Success in Testing". nytimes.com. Retrieved 16 June 2017.
- ↑ "RTS,S". malariavaccine.org. Retrieved 25 April 2017.
- ↑ "New malaria vaccine effective in clinical trial". sciencedaily.com. Retrieved 16 June 2017.
- ↑ "Malaria vaccine and HIV vaccine development". Google Trends. Retrieved 30 March 2021.
- ↑ "malaria vaccine, influenza vaccine, hiv vaccine, yellow fever vaccine and dengue vaccine". books.google.com. Retrieved 30 March 2021.
- ↑ "Malaria vaccine". wikipediaviews.org. Retrieved 17 March 2021.