Difference between revisions of "Timeline of nickel"
| (36 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
This is a '''timeline of {{w|nickel}}'''. One of the most widely used metals on the planet, nickel is used in over 300,000 different products. It is most often found in steels and metal alloys. It is also used in the production of batteries and permanent magnets.<ref name="Metal Profile: Nickel">{{cite web |title=Metal Profile: Nickel |url=https://www.thebalance.com/metal-profile-nickel-2340147 |website=thebalance.com |accessdate=9 August 2018}}</ref> | This is a '''timeline of {{w|nickel}}'''. One of the most widely used metals on the planet, nickel is used in over 300,000 different products. It is most often found in steels and metal alloys. It is also used in the production of batteries and permanent magnets.<ref name="Metal Profile: Nickel">{{cite web |title=Metal Profile: Nickel |url=https://www.thebalance.com/metal-profile-nickel-2340147 |website=thebalance.com |accessdate=9 August 2018}}</ref> | ||
| + | |||
| + | ==Sample questions== | ||
| + | |||
| + | The following are some interesting questions that can be answered by reading this timeline: | ||
| + | |||
| + | * When is the nickel date of discovery? | ||
| + | * How was nickel discovered? | ||
| + | * Where was nickel first discovered? | ||
| Line 7: | Line 15: | ||
! Time period !! Development summary | ! Time period !! Development summary | ||
|- | |- | ||
| − | | Ancient history || Nickel is already used industrially as an alloying metal almost 2,000 years before it is isolated and recognized as a new element.<ref name="Nickel processing britannica.com"/> | + | | Ancient history || Nickel is already used industrially as an alloying metal almost 2,000 years before it is isolated and recognized as a new element.<ref name="Nickel processing britannica.com"/> Its production sees an early development in China. |
|- | |- | ||
| 15th century || German miners, who believe they can extract {{w|copper}} from nickel ores in {{w|Saxony}}, refer to the metal as kupfernickel ("the devil's copper"), partly due to their futile attempts to extract copper from the ore, but also likely in part due to the health effects caused by the high arsenic content in the ore.<ref name="Metal Profile: Nickel"/> | | 15th century || German miners, who believe they can extract {{w|copper}} from nickel ores in {{w|Saxony}}, refer to the metal as kupfernickel ("the devil's copper"), partly due to their futile attempts to extract copper from the ore, but also likely in part due to the health effects caused by the high arsenic content in the ore.<ref name="Metal Profile: Nickel"/> | ||
| + | |- | ||
| + | | 18th century || Nickel is discovered in {{w|Sweden}} in this century, by {{w|Axel Fredrik Cronstedt}}. | ||
|- | |- | ||
| 19th century || Nickel's use in plating and alloys with copper and zinc begins.<ref name="THE HISTORY OF NICKEL TWIN METALS">{{cite web |title=THE HISTORY OF NICKEL |url=http://www.twin-metals.com/the-history-of-nickel/ |website=twin-metals.com |accessdate=9 August 2018}}</ref> Since the mid-century, nickel is the component of coins in the United States, India, Switzerland, and Canada.<?> | | 19th century || Nickel's use in plating and alloys with copper and zinc begins.<ref name="THE HISTORY OF NICKEL TWIN METALS">{{cite web |title=THE HISTORY OF NICKEL |url=http://www.twin-metals.com/the-history-of-nickel/ |website=twin-metals.com |accessdate=9 August 2018}}</ref> Since the mid-century, nickel is the component of coins in the United States, India, Switzerland, and Canada.<?> | ||
| Line 15: | Line 25: | ||
| 20th century || Early in the century, the discovery of large scale deposits in {{w|Russia}} and {{w|South Africa}} makes large-scale production of nickel possible. A significant increase in demand of the metal results from {{w|World War I}} and {{w|World War II}}.<ref name="Metal Profile: Nickel"/> Along with development in society, the nickel industry develops rapidly since 1974, and the metal plays an important role in the stainless steel industry, battery industry and certain other industries. Nickel consumption increases greatly between 1974 and 2011.<ref name="Nickel: discovery and industry development">{{cite web |title=Nickel: discovery and industry development |url=http://metalpedia.asianmetal.com/metal/nickel/history.shtml |website=metalpedia.asianmetal.com |accessdate=9 August 2018}}</ref> | | 20th century || Early in the century, the discovery of large scale deposits in {{w|Russia}} and {{w|South Africa}} makes large-scale production of nickel possible. A significant increase in demand of the metal results from {{w|World War I}} and {{w|World War II}}.<ref name="Metal Profile: Nickel"/> Along with development in society, the nickel industry develops rapidly since 1974, and the metal plays an important role in the stainless steel industry, battery industry and certain other industries. Nickel consumption increases greatly between 1974 and 2011.<ref name="Nickel: discovery and industry development">{{cite web |title=Nickel: discovery and industry development |url=http://metalpedia.asianmetal.com/metal/nickel/history.shtml |website=metalpedia.asianmetal.com |accessdate=9 August 2018}}</ref> | ||
|- | |- | ||
| − | | 21st century || As of 2010, the largest producers of nickel are Russia, Canada, Australia, and Indonesia. The largest producers of refined nickel are Norilsk Nickel, Vale S.A., and Jinchuan Group Ltd. Only a small percentage of nickel is produced from recycled materials,<ref name="Metal Profile: Nickel"/> however, nickel recycling industry is developing fast.<ref name="Nickel: discovery and industry development"/> | + | | 21st century || More than 1.4 million tons of primary or new nickel is produced worldwide.<ref name="NICKEL MINING AND PROCESSING: EVERYTHING YOU NEED TO KNOW"/> As of 2010, the largest producers of nickel are Russia, Canada, Australia, and Indonesia. The largest producers of refined nickel are Norilsk Nickel, Vale S.A., and Jinchuan Group Ltd. Only a small percentage of nickel is produced from recycled materials,<ref name="Metal Profile: Nickel"/> however, nickel recycling industry is developing fast.<ref name="Nickel: discovery and industry development"/> Nickel is now recognized as one of the most valuable mined materials, making nickel mining an important industry.<ref name="NICKEL MINING AND PROCESSING: EVERYTHING YOU NEED TO KNOW"/> |
|- | |- | ||
|} | |} | ||
| + | |||
| + | |||
==Full timeline== | ==Full timeline== | ||
| Line 24: | Line 36: | ||
! Year !! Event type !! Details !! Country/region | ! Year !! Event type !! Details !! Country/region | ||
|- | |- | ||
| − | | 3500 BC || || Syrian bronzes contain a small amount of nickel.<ref name="Nickel: discovery and industry development"/> || {{w|Syria}} | + | | 3500 BC || Application || Syrian bronzes contain a small amount of nickel.<ref name="Nickel: discovery and industry development"/> || {{w|Syria}} |
|- | |- | ||
| − | | 1700 BC – 1400 BC || || Chinese manuscripts suggest use of white copper (cupronickel).<ref name="Nickel: discovery and industry development"/> || {{w|China}} | + | | 1700 BC – 1400 BC || Application || Chinese manuscripts suggest use of white copper (cupronickel).<ref name="Nickel: discovery and industry development"/> || {{w|China}} |
|- | |- | ||
| − | | 1500 BC || || Chinese documents from that time make reference to "white copper" (baitong), whis is very likely an alloy of nickel and {{w|silver}}.<ref name="Metal Profile: Nickel"/> || {{w|China}} | + | | 1500 BC || Application || Chinese documents from that time make reference to "white copper" (baitong), whis is very likely an alloy of nickel and {{w|silver}}.<ref name="Metal Profile: Nickel"/> || {{w|China}} |
|- | |- | ||
| − | | 235 BC || || Coins in China are made of nickel.<ref name="Nickel: discovery and industry development"/> || {{w|China}} | + | | 235 BC || Application || Coins in China are made of nickel.<ref name="Nickel: discovery and industry development"/><ref name="NICKEL MINING AND PROCESSING: EVERYTHING YOU NEED TO KNOW"/><ref>{{cite web |title=Ni - Nickel |url=http://eperiodictable.com/Ni.html |website=eperiodictable.com |accessdate=25 August 2018}}</ref><ref name="Metal Story: Nickel (Ni)"/> || {{w|China}} |
|- | |- | ||
| − | | 200 BC || || The Chinese make substantial amounts of a white alloy from zinc and copper-nickel ore found in Yunnan province. The alloy, known as ''pai-t'ung'', is exported to the {{w|Middle East}} and even to {{w|Europe}}.<ref name="Nickel processing britannica.com"/> || {{w|China}} | + | | 200 BC || Application || The Chinese make substantial amounts of a white alloy from zinc and copper-nickel ore found in Yunnan province. The alloy, known as ''pai-t'ung'', is exported to the {{w|Middle East}} and even to {{w|Europe}}.<ref name="Nickel processing britannica.com"/> || {{w|China}} |
|- | |- | ||
| − | | 1751 || || Swedish chemist Axel Fredrik Cronstedt first extracts pure nickel from the mineral niccolite (NiAs).<ref name="Metal Profile: Nickel"/><ref name="THE HISTORY OF NICKEL TWIN METALS"/> || | + | | 1751 || Scientific development || Swedish chemist Axel Fredrik Cronstedt first extracts pure nickel from the mineral niccolite (NiAs).<ref name="Metal Profile: Nickel"/><ref name="THE HISTORY OF NICKEL TWIN METALS"/><ref name="Metal Story: Nickel (Ni)"/> Expecting to extract {{w|copper}} from this mineral, Cronsted obtains instead a white metal that he ends up calling nickel after the mineral from which it was extracted.<ref>{{cite web |title=Nickel: historical information |url=https://www.webelements.com/nickel/history.html |website=webelements.com |accessdate=30 November 2019}}</ref> || {{w|Sweden}} |
|- | |- | ||
| − | | 1775 || || Swedish mineralogist Torbern Bergman first fully purifies nickel.<ref>{{cite book |last1=Holleman |first1=A. F. |last2=Wiberg |first2=Egon |last3=Wiberg |first3=Nils |title=Inorganic Chemistry |url=https://books.google.com.ar/books?id=Mtth5g59dEIC&pg=PA1500&lpg=PA1500&dq=%22in+1775%22+%22Torbern+Bergman%22+%22nickel%22&source=bl&ots=tFXNfzL2Py&sig=-EH16eVobpZrc-OcGAjLCurAcM4&hl=en&sa=X&ved=2ahUKEwjNqdOBmofdAhWEjpAKHbHhAZEQ6AEwAXoECAkQAQ#v=onepage&q=%22in%201775%22%20%22Torbern%20Bergman%22%20%22nickel%22&f=false}}</ref><ref>{{cite web |title=What in the Word?! The bedeviling origins of ‘nickel’ |url=https://blog.oxforddictionaries.com/2017/12/23/what-in-the-word-nickel/ |website=blog.oxforddictionaries.com |accessdate=25 August 2018}}</ref><ref>{{cite book |last1=Morris |first1=Richard |title=The Last Sorcerers: The Path from Alchemy to the Periodic Table |url=https://books.google.com.ar/books?id=4X6tYQr2r6IC&pg=PT91&lpg=PT91&dq=%22in+1775%22+%22Torbern+Bergman%22+%22nickel%22&source=bl&ots=YxexUI_Vle&sig=xmkV1DoWUHrfOsf0MoHB8lFPY2U&hl=en&sa=X&ved=2ahUKEwjNqdOBmofdAhWEjpAKHbHhAZEQ6AEwAnoECAgQAQ#v=onepage&q=%22in%201775%22%20%22Torbern%20Bergman%22%20%22nickel%22&f=false}}</ref> || | + | | 1775 || Scientific development || Swedish mineralogist Torbern Bergman first fully purifies nickel.<ref>{{cite book |last1=Holleman |first1=A. F. |last2=Wiberg |first2=Egon |last3=Wiberg |first3=Nils |title=Inorganic Chemistry |url=https://books.google.com.ar/books?id=Mtth5g59dEIC&pg=PA1500&lpg=PA1500&dq=%22in+1775%22+%22Torbern+Bergman%22+%22nickel%22&source=bl&ots=tFXNfzL2Py&sig=-EH16eVobpZrc-OcGAjLCurAcM4&hl=en&sa=X&ved=2ahUKEwjNqdOBmofdAhWEjpAKHbHhAZEQ6AEwAXoECAkQAQ#v=onepage&q=%22in%201775%22%20%22Torbern%20Bergman%22%20%22nickel%22&f=false}}</ref><ref>{{cite web |title=What in the Word?! The bedeviling origins of ‘nickel’ |url=https://blog.oxforddictionaries.com/2017/12/23/what-in-the-word-nickel/ |website=blog.oxforddictionaries.com |accessdate=25 August 2018}}</ref><ref>{{cite book |last1=Morris |first1=Richard |title=The Last Sorcerers: The Path from Alchemy to the Periodic Table |url=https://books.google.com.ar/books?id=4X6tYQr2r6IC&pg=PT91&lpg=PT91&dq=%22in+1775%22+%22Torbern+Bergman%22+%22nickel%22&source=bl&ots=YxexUI_Vle&sig=xmkV1DoWUHrfOsf0MoHB8lFPY2U&hl=en&sa=X&ved=2ahUKEwjNqdOBmofdAhWEjpAKHbHhAZEQ6AEwAnoECAgQAQ#v=onepage&q=%22in%201775%22%20%22Torbern%20Bergman%22%20%22nickel%22&f=false}}</ref> || |
|- | |- | ||
| − | | 1776 || || It is established that the Chinese alloy ''pai-t'ung'', now called nickel-silver, is composed of {{w|copper}}, nickel, and {{w|zinc}}.<ref name="Nickel processing britannica.com"/> || | + | | 1776 || Application || It is established that the Chinese alloy ''pai-t'ung'', now called nickel-silver, is composed of {{w|copper}}, nickel, and {{w|zinc}}.<ref name="Nickel processing britannica.com"/> || |
|- | |- | ||
| − | | 1796 || || William Nicholson publishes First Principles of Chemistry. About nickel, Nicholson writes: | + | | 1796 || Scientific development || English chemist [[w:William Nicholson (chemist)|William Nicholson]] publishes First Principles of Chemistry. About nickel, Nicholson writes: |
This metallic substance has not been applied to any use; and the chief attention of those chemists who have examined it has been directed to obtain it in a state of purity; which however has not been accomplished... Nickel has been thought to be a modification of iron... So long as no one is able to produce this metal from pure iron or copper, and to explain in an intelligible way the process by which it can be generated, we must continue to regard it as a peculiar substance, possessing distinct properties. The general opinions of chemists concur in admitting the force of this reasoning.<ref>{{cite book |last1=Enghag |first1=Per |title=Encyclopedia of the Elements: Technical Data - History - Processing - Applications |url=https://books.google.com.ar/books?id=fUmTX8yKU4gC&pg=PA690&lpg=PA690&dq=%22in+1775%22+%22Torbern+Bergman%22+%22nickel%22&source=bl&ots=JLRKqOXil_&sig=3y9YUcQqQTFey3nw0M9O9wtspiU&hl=en&sa=X&ved=2ahUKEwjNqdOBmofdAhWEjpAKHbHhAZEQ6AEwBHoECAQQAQ#v=onepage&q=%22in%201775%22%20%22Torbern%20Bergman%22%20%22nickel%22&f=false}}</ref> | This metallic substance has not been applied to any use; and the chief attention of those chemists who have examined it has been directed to obtain it in a state of purity; which however has not been accomplished... Nickel has been thought to be a modification of iron... So long as no one is able to produce this metal from pure iron or copper, and to explain in an intelligible way the process by which it can be generated, we must continue to regard it as a peculiar substance, possessing distinct properties. The general opinions of chemists concur in admitting the force of this reasoning.<ref>{{cite book |last1=Enghag |first1=Per |title=Encyclopedia of the Elements: Technical Data - History - Processing - Applications |url=https://books.google.com.ar/books?id=fUmTX8yKU4gC&pg=PA690&lpg=PA690&dq=%22in+1775%22+%22Torbern+Bergman%22+%22nickel%22&source=bl&ots=JLRKqOXil_&sig=3y9YUcQqQTFey3nw0M9O9wtspiU&hl=en&sa=X&ved=2ahUKEwjNqdOBmofdAhWEjpAKHbHhAZEQ6AEwBHoECAQQAQ#v=onepage&q=%22in%201775%22%20%22Torbern%20Bergman%22%20%22nickel%22&f=false}}</ref> | ||
| − | || | + | || {{w|United Kingdom}} |
| + | |- | ||
| + | | 1820 || Application || British chemist and natural philosopher {{w|Michael Faraday}} carries out several experiments in making nickel-containing steels, but fails to interest the steelmakers.<ref name="Metal Story: Nickel (Ni)">{{cite web |title=Metal Story: Nickel (Ni) |url=https://oristarcorp.com/en/metal-story-nickel-ni/ |website=oristarcorp.com |accessdate=25 August 2018}}</ref> || {{w|United Kingdom}} | ||
|- | |- | ||
| − | | 1837 || || G. Bird describes electrodeposition of nickel chloride or sulphate on platinum. This process results in a thin crust of nickel on the platinum substrate.<ref name="The History of Nickel Plating">{{cite web |title=The History of Nickel Plating |url=https://www.thomasnet.com/articles/metals-metal-products/nickel-plating-history |website=thomasnet.com |accessdate=9 August 2018}}</ref><ref>{{cite book |last1=Dennis |first1=J. K. |last2=Such |first2=T. E. |title=Nickel and Chromium Plating |url=https://books.google.com.ar/books?id=Kgz-BAAAQBAJ&pg=PA3&lpg=PA3&dq=%22in+1837%22+G.+Bird+describes+electrodeposition+of+nickel&source=bl&ots=CbIvpdmIcM&sig=f8cNgZRgB3EpUPLjlOQn8Ho4atE&hl=en&sa=X&ved=2ahUKEwiv7o6wtojdAhVBlJAKHSkOCd0Q6AEwAHoECAEQAQ#v=onepage&q=%22in%201837%22%20G.%20Bird%20describes%20electrodeposition%20of%20nickel&f=false}}</ref><ref>{{cite journal |last1=Wang |first1=J |last2=Gabe |first2=D.R. |title=The chemistry of nickel electroplating solutions |url=https://www.tandfonline.com/doi/abs/10.1179/0020296712Z.00000000079?scroll=top&needAccess=true&journalCode=ytim20}}</ref> || | + | | 1837 || Application || G. Bird describes electrodeposition of nickel chloride or sulphate on platinum. This process results in a thin crust of nickel on the platinum substrate.<ref name="The History of Nickel Plating">{{cite web |title=The History of Nickel Plating |url=https://www.thomasnet.com/articles/metals-metal-products/nickel-plating-history |website=thomasnet.com |accessdate=9 August 2018}}</ref><ref>{{cite book |last1=Dennis |first1=J. K. |last2=Such |first2=T. E. |title=Nickel and Chromium Plating |url=https://books.google.com.ar/books?id=Kgz-BAAAQBAJ&pg=PA3&lpg=PA3&dq=%22in+1837%22+G.+Bird+describes+electrodeposition+of+nickel&source=bl&ots=CbIvpdmIcM&sig=f8cNgZRgB3EpUPLjlOQn8Ho4atE&hl=en&sa=X&ved=2ahUKEwiv7o6wtojdAhVBlJAKHSkOCd0Q6AEwAHoECAEQAQ#v=onepage&q=%22in%201837%22%20G.%20Bird%20describes%20electrodeposition%20of%20nickel&f=false}}</ref><ref>{{cite journal |last1=Wang |first1=J |last2=Gabe |first2=D.R. |title=The chemistry of nickel electroplating solutions |url=https://www.tandfonline.com/doi/abs/10.1179/0020296712Z.00000000079?scroll=top&needAccess=true&journalCode=ytim20}}</ref> || |
|- | |- | ||
| − | | | + | | 1840 || Application || Joseph Shore applies for a patent for nickel plating.<ref name="Nickel Plating Primer">{{cite web |title=Nickel Plating Primer |url=https://www.pfonline.com/articles/nickel-plating-primer |website=pfonline.com |accessdate=25 August 2018}}</ref> || |
|- | |- | ||
| − | | 1860 || || An alloy of 25% nickel and 75% copper, essentially white in color, is adopted for coinage in Belgium.<ref name="Nickel processing britannica.com"/> || {{w|Belgium}} | + | | 1842 || Application || Böttger in {{w|Frankfurt}} succeeds in depositing nickel from a solution of nickel ammonium sulfate.<ref name="Nickel Plating Primer"/> || {{w|Germany}} |
| + | |- | ||
| + | | 1844 || Production || Demand of nickel-silver is stimulated in {{w|England}} by the development of silver electroplating, for which it is found to be the most desirable base. The use of pure nickel as a corrosion-resistant electroplated coating would develop a little later.<ref name="Nickel processing britannica.com"/> || {{w|United Kingdom}} | ||
| + | |- | ||
| + | | 1860 || Application || An alloy of 25% nickel and 75% copper, essentially white in color, is adopted for coinage in Belgium.<ref name="Nickel processing britannica.com"/> || {{w|Belgium}} | ||
|- | |- | ||
| 1865 || Application || An alloy copper-nickel is adopted for coinage in the United States.<ref name="Nickel processing britannica.com"/> || {{w|United States}} | | 1865 || Application || An alloy copper-nickel is adopted for coinage in the United States.<ref name="Nickel processing britannica.com"/> || {{w|United States}} | ||
|- | |- | ||
| − | | 1869 || Application || Dr I. Adams in the United States patents a nickel ammonium sulphate bath.<ref name="The History of Nickel Plating"/> || {{w|United States}} | + | | 1865 || Reserve || Big deposits of nickel ores are discovered in New Caledonia.<ref name="Metal Story: Nickel (Ni)"/> || {{w|New Caledonia}} |
| + | |- | ||
| + | | 1868 || Application || W.H. Remington from {{w|Boston}} commences the deposition of nickel on a commercial scale using a nickel ammonium chloride solution.<ref name="Nickel Plating Primer"/><ref name="Nickel and Chromium Plating">{{cite book |last1=Dennis |first1=J. K. |last2=Such |first2=T. E. |title=Nickel and Chromium Plating |url=https://books.google.com.ar/books?id=Kgz-BAAAQBAJ&pg=PA4&lpg=PA4&dq=%22in+1869%22+nickel+ammonium&source=bl&ots=CbIvpdnBkN&sig=WtrAcyf6tOn95gWttA-eIkmrCFQ&hl=en&sa=X&ved=2ahUKEwi-qvrlt4jdAhUBkJAKHYPlBSEQ6AEwAnoECAgQAQ#v=onepage&q=%22in%201869%22%20nickel%20ammonium&f=false}}</ref> || {{w|United States}} | ||
| + | |- | ||
| + | | 1869 || Application || Dr I. Adams in the United States patents a nickel ammonium sulphate bath.<ref>{{cite book |title=Popular Science Aug 1884 |url=https://books.google.com.ar/books?id=mCwDAAAAMBAJ&pg=PA569&lpg=PA569&dq=%22in+1869%22+nickel+ammonium&source=bl&ots=F1fZnOBJtd&sig=PkJptToigT_QxxIVI558UnhZyzE&hl=en&sa=X&ved=2ahUKEwi-qvrlt4jdAhUBkJAKHYPlBSEQ6AEwBHoECAUQAQ#v=onepage&q=%22in%201869%22%20nickel%20ammonium&f=false}}</ref><ref name="The History of Nickel Plating"/><ref name="Nickel Plating Primer"/><ref name="Nickel and Chromium Plating"/><ref name="A Retrospective View of Nickel Plating"/><ref>{{cite web |title=Week 31 |url=https://www.acs.org/content/acs/en/education/whatischemistry/this-week-in-chemical-history/week-31.html |website=acs.org |accessdate=25 August 2018}}</ref> || {{w|United States}} | ||
| + | |- | ||
| + | | 1877 || Production || Production of nickel starts in {{w|New Caledonia}} in the South Pacific.<ref name="Nickel processing britannica.com"/><ref>{{cite web |title=Nickel - Mining and metallurgy |url=https://www.croixdusud.info/econom_eng/niexploit_eng.php |website=croixdusud.info |accessdate=25 August 2018}}</ref> || {{w|New Caledonia}} | ||
|- | |- | ||
| − | | | + | | 1878 || Application || Weston obtains a patent for the addition of boric acid to nickel plating solutions.<ref name="Nickel Plating Primer"/> || |
| + | |- | ||
| + | | 1880 || Application || Nickel plating salts are being made in {{w|Vienna}} by Pfanhauser and in {{w|Birmingham}} by {{w|Canning}}.<ref name="Nickel and Chromium Plating"/> || {{w|Austria}}, {{w|United Kingdom}} | ||
|- | |- | ||
| 1881 || Application || Coins of nearly pure nickel are first used in Switzerland.<ref name="Nickel: discovery and industry development"/><ref name="Nickel processing britannica.com"/> || {{w|Switzerland}} | | 1881 || Application || Coins of nearly pure nickel are first used in Switzerland.<ref name="Nickel: discovery and industry development"/><ref name="Nickel processing britannica.com"/> || {{w|Switzerland}} | ||
|- | |- | ||
| − | | 1889 || || British engineer James Riley makes a presentation to the Iron and Steel Institute of Great Britain on how the introduction of nickel could strenghten traditional steels. Riley's presentation results in a growing awareness of nickel's beneficial alloying properties and coincides with the discovery of large nickel deposits in New Caledonia and Canada.<ref name="Metal Profile: Nickel"/><ref>{{cite book |last1=Sparrow |first1=Giles |title=Nickel |url=https://books.google.com.ar/books?id=lgIp8ZTaYXAC&pg=PA20&lpg=PA20&dq=%22in+1889%22+%22James+Riley%22+%22nickel%22&source=bl&ots=BN4FVz5cDn&sig=eCxGs1yEYTp6-DiPcXqgrYfpcuM&hl=en&sa=X&ved=2ahUKEwjLnuXMjYfdAhUGHJAKHe2GBxwQ6AEwC3oECAcQAQ#v=onepage&q=%22in%201889%22%20%22James%20Riley%22%20%22nickel%22&f=false}}</ref><ref name="Metallurgy: 1863-1963">{{cite book |last1=Dennis |first1=W.H. |title=Metallurgy: 1863-1963 |url=https://books.google.com.ar/books?id=gSEuDwAAQBAJ&pg=PT151&lpg=PT151&dq=%22in+1889%22+%22James+Riley%22+%22nickel%22&source=bl&ots=TSTCIK6t7G&sig=_GZsCPW19ZUz39Z6lD8N7Hl2UOg&hl=en&sa=X&ved=2ahUKEwjLnuXMjYfdAhUGHJAKHe2GBxwQ6AEwDHoECAUQAQ#v=onepage&q=%22in%201889%22%20%22James%20Riley%22%20%22nickel%22&f=false}}</ref> || {{w|United Kingdom}} | + | | 1889 || Application || British engineer James Riley makes a presentation to the Iron and Steel Institute of Great Britain on how the introduction of nickel could strenghten traditional steels. Riley's presentation results in a growing awareness of nickel's beneficial alloying properties and coincides with the discovery of large nickel deposits in New Caledonia and Canada.<ref name="Metal Profile: Nickel"/><ref>{{cite book |last1=Sparrow |first1=Giles |title=Nickel |url=https://books.google.com.ar/books?id=lgIp8ZTaYXAC&pg=PA20&lpg=PA20&dq=%22in+1889%22+%22James+Riley%22+%22nickel%22&source=bl&ots=BN4FVz5cDn&sig=eCxGs1yEYTp6-DiPcXqgrYfpcuM&hl=en&sa=X&ved=2ahUKEwjLnuXMjYfdAhUGHJAKHe2GBxwQ6AEwC3oECAcQAQ#v=onepage&q=%22in%201889%22%20%22James%20Riley%22%20%22nickel%22&f=false}}</ref><ref name="Metallurgy: 1863-1963">{{cite book |last1=Dennis |first1=W.H. |title=Metallurgy: 1863-1963 |url=https://books.google.com.ar/books?id=gSEuDwAAQBAJ&pg=PT151&lpg=PT151&dq=%22in+1889%22+%22James+Riley%22+%22nickel%22&source=bl&ots=TSTCIK6t7G&sig=_GZsCPW19ZUz39Z6lD8N7Hl2UOg&hl=en&sa=X&ved=2ahUKEwjLnuXMjYfdAhUGHJAKHe2GBxwQ6AEwDHoECAUQAQ#v=onepage&q=%22in%201889%22%20%22James%20Riley%22%20%22nickel%22&f=false}}</ref> || {{w|United Kingdom}} |
|- | |- | ||
| − | | 1890 || || {{w|Nickel tetracarbonyl}} Ni(CO)<sub>4</sub>, is discovered. This compound, in which nickel exhibits a zero oxidation state, is used primarily as a carrier of carbon monoxide in the synthesis of acrylates (compounds used in the manufacture of plastics) from acetylene and alcohols. Nickel carbonyl is the first of a class of compounds called metal carbonyls to be discovered.<ref name="Nickel britannica">{{cite web |title=Nickel |url=https://www.britannica.com/science/nickel-chemical-element |website=britannica.com |accessdate=9 August 2018}}</ref> || | + | | 1890 || Scientific development || {{w|Nickel tetracarbonyl}} Ni(CO)<sub>4</sub>, is discovered. This compound, in which nickel exhibits a zero oxidation state, is used primarily as a carrier of carbon monoxide in the synthesis of acrylates (compounds used in the manufacture of plastics) from acetylene and alcohols. Nickel carbonyl is the first of a class of compounds called metal carbonyls to be discovered.<ref name="Nickel britannica">{{cite web |title=Nickel |url=https://www.britannica.com/science/nickel-chemical-element |website=britannica.com |accessdate=9 August 2018}}</ref> || |
|- | |- | ||
| − | | 1890 || || A paper entitled ''Action of Carbon Monoxide on Nickel'', by Ludwig Mond, Carl Langer and Friedrich Qpincke ,describes the formation of a gaseous compound of nickel and carbon monoxide by the reaction of the latter with finely divided nickel.<ref name="Metallurgy: 1863-1963"/> || {{w|United Kingdom}} | + | | 1890 || Scientific development || A paper entitled ''Action of Carbon Monoxide on Nickel'', by Ludwig Mond, Carl Langer and Friedrich Qpincke, describes the formation of a gaseous compound of nickel and carbon monoxide by the reaction of the latter with finely divided nickel.<ref name="Metallurgy: 1863-1963"/> || {{w|United Kingdom}} |
|- | |- | ||
| − | | 1898 || || Invar, an alloy containing 36% nickel, is discovered. With the balance iron, invar is notable for its extremely small thermal expansion. Along with later-developed nickel alloys, invar would have many applications ranging from thermostats to balance wheels for watches, metal-to-glass seals essential to electric lights, and radio tubes.<ref name="Nickel processing britannica.com"/><ref>{{cite book |title=Instruments & Control Systems, Volume 13 |publisher=Chilton Company, 1940 |url=https://books.google.com.ar/books?id=Eg8nAAAAMAAJ&q=%22in+1898%22+%22Invar%22+%22nickel%22&dq=%22in+1898%22+%22Invar%22+%22nickel%22&hl=en&sa=X&ved=0ahUKEwj-msKblIfdAhWTnJAKHZhkC944ChDoAQgnMAA | + | | 1898 || Application || Invar, an alloy containing 36% nickel, is discovered. With the balance iron, invar is notable for its extremely small thermal expansion. Along with later-developed nickel alloys, invar would have many applications ranging from thermostats to balance wheels for watches, metal-to-glass seals essential to electric lights, and radio tubes.<ref name="Nickel processing britannica.com"/><ref>{{cite book |title=Instruments & Control Systems, Volume 13 |publisher=Chilton Company, 1940 |url=https://books.google.com.ar/books?id=Eg8nAAAAMAAJ&q=%22in+1898%22+%22Invar%22+%22nickel%22&dq=%22in+1898%22+%22Invar%22+%22nickel%22&hl=en&sa=X&ved=0ahUKEwj-msKblIfdAhWTnJAKHZhkC944ChDoAQgnMAA}}</ref><ref>{{cite web |title=Invar |url=https://www.britannica.com/technology/Invar |website=britannica.com |accessdate=25 August 2018}}</ref> || |
|- | |- | ||
| − | | 1899 || || W. Browne, of the Canadian Copper Co., develops an electrolytic process for the separation of nickel and copper.<ref name="Metallurgy: 1863-1963"/> || {{w|Canada}} | + | | 1899 || Technology || W. Browne, of the Canadian Copper Co., develops an electrolytic process for the separation of nickel and copper.<ref name="Metallurgy: 1863-1963"/> || {{w|Canada}} |
|- | |- | ||
| − | | | + | | 1899 || Technology || German-born British chemist {{w|Ludwig Mond}} develops the now called Mond Process, for extraction and purification of nickel.<ref name="NICKEL MINING AND PROCESSING: EVERYTHING YOU NEED TO KNOW">{{cite web |title=NICKEL MINING AND PROCESSING: EVERYTHING YOU NEED TO KNOW |url=https://www.generalkinematics.com/blog/nickel-mining-processing-everything-need-know/ |website=generalkinematics.com |accessdate=25 August 2018}}</ref> || {{w|United Kingdom}} |
|- | |- | ||
| − | | 1905 || || The Copper Cliff-Sudbury, Ontario region in Canada becomes the world's largest source of nickel.<ref name="Nickel processing britannica.com">{{cite web |title=Nickel processing |url=https://www.britannica.com/technology/nickel-processing |website=britannica.com |accessdate=9 August 2018}}</ref> || {{w|Canada}} | + | | 1901 || Technology || Thomas Edison is awarded a patent por nickel-zinc (NiZn) chemistry. However, Edison would never be able to commercialize NiZn products. NiZn electrochemistry is found to have great potential because of its high energy density, high efficiency rating and temperature tolerance advantages compared to other battery chemistries.<ref name="Nickel-Zinc history zincfive"/> || {{w|United States}} |
| + | |- | ||
| + | | 1905 || Reserve || The Copper Cliff-Sudbury, Ontario region in Canada becomes the world's largest source of nickel.<ref name="Nickel processing britannica.com">{{cite web |title=Nickel processing |url=https://www.britannica.com/technology/nickel-processing |website=britannica.com |accessdate=9 August 2018}}</ref> || {{w|Canada}} | ||
| + | |- | ||
| + | | 1915 || Scientific development || An investigation by H.T. Kalmus and coworkers dealing with cobalt as well as nickel is summarized in the publication ''Transactions of the Electrochemical Society''. This work leads the authors to the opinion that nickel could not be plated under commercial conditions at current densities as high as those possible for cobalt.<ref name="A Retrospective View of Nickel Plating"/> || | ||
| + | |- | ||
| + | | 1916 || Application || O.P. Watts develops a rapid nickel plating bath.<ref name="Nickel Plating Primer"/> || | ||
| + | |- | ||
| + | | 1918 || Production || The refining of nickel to the finished metal product begins in Canada with the building of Inco's refinery at Port Colborne, Ontario, using hydroelectric power from {{w|Niagara Falls}}.<ref>{{cite web |title=Nickel |url=https://uwaterloo.ca/earth-sciences-museum/resources/detailed-rocks-and-minerals-articles/nickel |website=uwaterloo.ca |accessdate=25 August 2018}}</ref> || {{w|Canada}} | ||
| + | |- | ||
| + | | 1921 || Technology || The use of {{w|pH}} measurements for controlling nickel baths is first suggested. By the end of the decade, it would become a common industrial practice.<ref name="Nickel and Chromium Plating"/> || | ||
| + | |- | ||
| + | | 1924 || Application || Nickel is used to plate automobile bumpers.<ref name="A Retrospective View of Nickel Plating">{{cite web |title=A Retrospective View of Nickel Plating |url=https://www.pfonline.com/articles/a-retrospective-view-of-nickel-plating |website=pfonline.com |accessdate=25 August 2018}}</ref> || | ||
|- | |- | ||
| 1930 – 1935 || Application || Nickel-containing permanent-magnet alloys are developed in Japan.<ref name="Nickel processing britannica.com"/> || {{w|Japan}} | | 1930 – 1935 || Application || Nickel-containing permanent-magnet alloys are developed in Japan.<ref name="Nickel processing britannica.com"/> || {{w|Japan}} | ||
| + | |- | ||
| + | | 1942 || Technology || W.A. Wesley and E.J. Roehl describe a bath of nickel sulfate, ammonium chloride and boric acid that could be operated at high pH to produce deposits with hardness in the range of 350-500 Vickers and tensile strength around 150,000 psi.<ref name="A Retrospective View of Nickel Plating"/> || | ||
| + | |- | ||
| + | | 1945 – 1955 || Application || Research by the International Nickel Company, Inc. (Inco) demonstrates the efficacy of multilayer coatings in enhancing corrosion protection of plated nickel.<ref name="A Retrospective View of Nickel Plating"/> || | ||
| + | |- | ||
| + | | 1959 || Technology || George Gutzeit, one of the inventors of the Kanigen process, presents a comprehensive discussion of the chemistry of the catalytic nickel process.<ref name="A Retrospective View of Nickel Plating"/><ref>{{cite web |title=Historical Highlights of Electroless Plating |url=https://www.pfonline.com/articles/historical-highlights-of-electroless-plating |website=pfonline.com |accessdate=25 August 2018}}</ref> || | ||
|- | |- | ||
| 1970 – 1975 || Application || The nickel-hydrogen battery is proposed as the leading candidate for rechargeable aerospace batteries.<ref name="A History of Nickel Hydrogen Batteries">{{cite web |title=A History of Nickel Hydrogen Batteries |url=https://www.electrochem.org/dl/ma/201/pdfs/0252.pdf |website=electrochem.org |accessdate=9 August 2018}}</ref> || | | 1970 – 1975 || Application || The nickel-hydrogen battery is proposed as the leading candidate for rechargeable aerospace batteries.<ref name="A History of Nickel Hydrogen Batteries">{{cite web |title=A History of Nickel Hydrogen Batteries |url=https://www.electrochem.org/dl/ma/201/pdfs/0252.pdf |website=electrochem.org |accessdate=9 August 2018}}</ref> || | ||
| Line 78: | Line 122: | ||
| c.1979 || Production || Nickel production in {{w|Soviet Russia}} surpasses that in Canada.<ref name="Nickel processing britannica.com"/> || {{w|Russia}} | | c.1979 || Production || Nickel production in {{w|Soviet Russia}} surpasses that in Canada.<ref name="Nickel processing britannica.com"/> || {{w|Russia}} | ||
|- | |- | ||
| − | | 1993 || || {{w|Stanford University}} chemist Morris Eisenberg creates a new electrolyte that enables a significant increase in the NiZn battery cycle life.<ref name="Nickel-Zinc history zincfive">{{cite web |title=History |url=http://www.zincfive.com/nickelzinc-history/ |website=zincfive.com |accessdate=9 August 2018}}</ref> || {{w|United States}} | + | | 1980 || Application || Falconbridge Nickel Co. and Inco introduce round forms of pure electrolytic nickel for plating.<ref name="A Retrospective View of Nickel Plating"/> || |
| + | |- | ||
| + | | 1993 || Technology || {{w|Stanford University}} chemist Morris Eisenberg creates a new electrolyte that enables a significant increase in the NiZn battery cycle life.<ref name="Nickel-Zinc history zincfive">{{cite web |title=History |url=http://www.zincfive.com/nickelzinc-history/ |website=zincfive.com |accessdate=9 August 2018}}</ref> || {{w|United States}} | ||
|- | |- | ||
| 1998 (December) || Financial || Nickel trades at a record low of 3730.50.<ref name="Nickel reaches an all time high of 54050">{{cite web |title=Nickel |url=https://tradingeconomics.com/commodity/nickel |website=tradingeconomics.com |accessdate=9 August 2018}}</ref> || | | 1998 (December) || Financial || Nickel trades at a record low of 3730.50.<ref name="Nickel reaches an all time high of 54050">{{cite web |title=Nickel |url=https://tradingeconomics.com/commodity/nickel |website=tradingeconomics.com |accessdate=9 August 2018}}</ref> || | ||
| Line 87: | Line 133: | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | == Numerical and visual data == | ||
| + | |||
| + | === Google Scholar === | ||
| + | |||
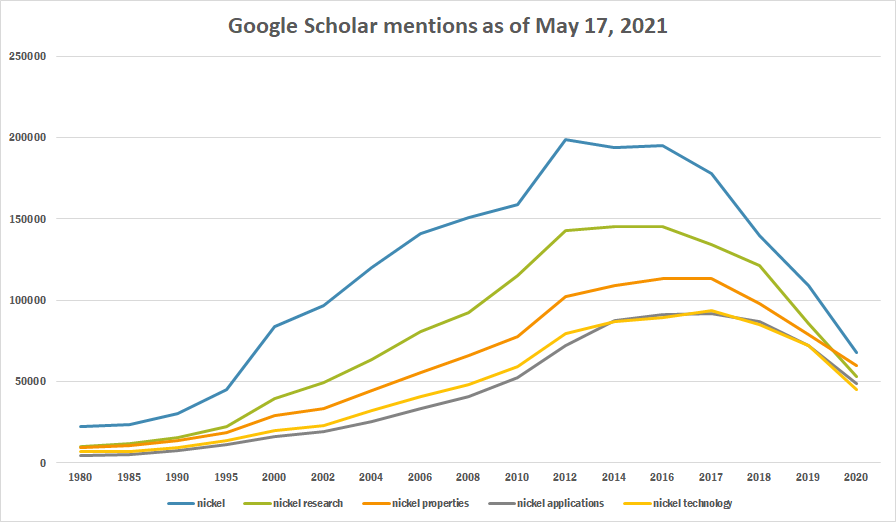
| + | The table below summarizes per-year mentions on Google Scholar as of May 17, 2021. | ||
| + | |||
| + | {| class="sortable wikitable" | ||
| + | ! Year | ||
| + | ! nickel | ||
| + | ! nickel research | ||
| + | ! nickel properties | ||
| + | ! nickel applications | ||
| + | ! nickel technology | ||
| + | |- | ||
| + | | 1980 || 22,100 || 10,100 || 9,640 || 4,610 || 7,010 | ||
| + | |- | ||
| + | | 1985 || 23,600 || 11,700 || 10,300 || 5,070 || 6,840 | ||
| + | |- | ||
| + | | 1990 || 30,300 || 15,400 || 13,800 || 7,490 || 9,370 | ||
| + | |- | ||
| + | | 1995 || 45,300 || 22,500 || 18,300 || 11,300 || 13,900 | ||
| + | |- | ||
| + | | 2000 || 83,700 || 39,600 || 28,800 || 16,400 || 19,900 | ||
| + | |- | ||
| + | | 2002 || 96,800 || 49,400 || 33,600 || 18,900 || 22,900 | ||
| + | |- | ||
| + | | 2004 || 120,000 || 63,600 || 44,600 || 25,300 || 32,000 | ||
| + | |- | ||
| + | | 2006 || 141,000 || 80,500 || 55,600 || 33,300 || 40,700 | ||
| + | |- | ||
| + | | 2008 || 151,000 || 92,600 || 66,100 || 40,900 || 48,100 | ||
| + | |- | ||
| + | | 2010 || 159,000 || 115,000 || 77,700 || 52,700 || 58,900 | ||
| + | |- | ||
| + | | 2012 || 199,000 || 143,000 || 102,000 || 71,800 || 79,200 | ||
| + | |- | ||
| + | | 2014 || 194,000 || 145,000 || 109,000 || 87,200 || 87,000 | ||
| + | |- | ||
| + | | 2016 || 195,000 || 145,000 || 113,000 || 91,100 || 89,600 | ||
| + | |- | ||
| + | | 2017 || 178,000 || 134,000 || 113,000 || 92,000 || 93,800 | ||
| + | |- | ||
| + | | 2018 || 140,000 || 121,000 || 97,700 || 86,800 || 84,700 | ||
| + | |- | ||
| + | | 2019 || 109,000 || 85,800 || 78,600 || 71,800 || 72,100 | ||
| + | |- | ||
| + | | 2020 || 67,900 || 52,800 || 59,600 || 48,800 || 44,800 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | [[File:Nickel tb.png|thumb|center|700px]] | ||
| + | |||
| + | === Google Trends === | ||
| + | |||
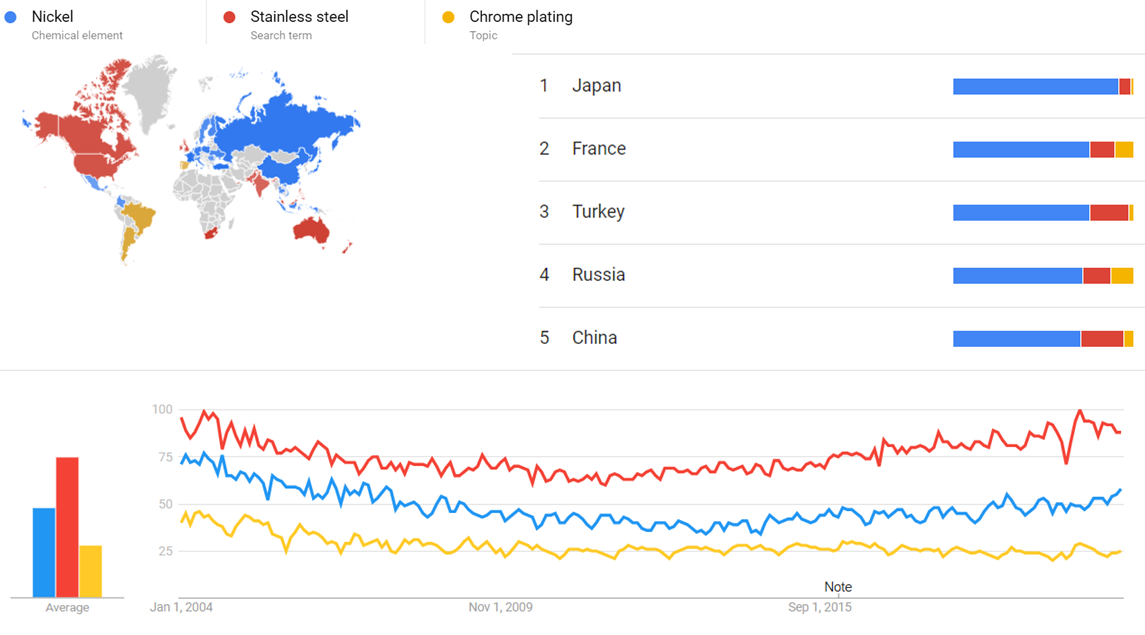
| + | The comparative chart below shows {{w|Google Trends}} data for Nickel (Chemical element), Stainless steel (Search Term) and Chrome plating (Topic), from January 2004 to March 2021, when the screenshot was taken. Interest is also ranked by country and displayed on world map.<ref>{{cite web |title=Nickel, Stainless steel and Chrome plating |url=https://trends.google.com/trends/explore?date=all&q=%2Fm%2F025s4r7,Stainless%20steel,%2Fm%2F02kqgg |website=Google Trends |access-date=22 March 2021}}</ref> | ||
| + | |||
| + | [[File:Nickel, Stainless steel and chrome plating gt.png|thumb|center|600px]] | ||
| + | |||
| + | === Google Ngram Viewer === | ||
| + | |||
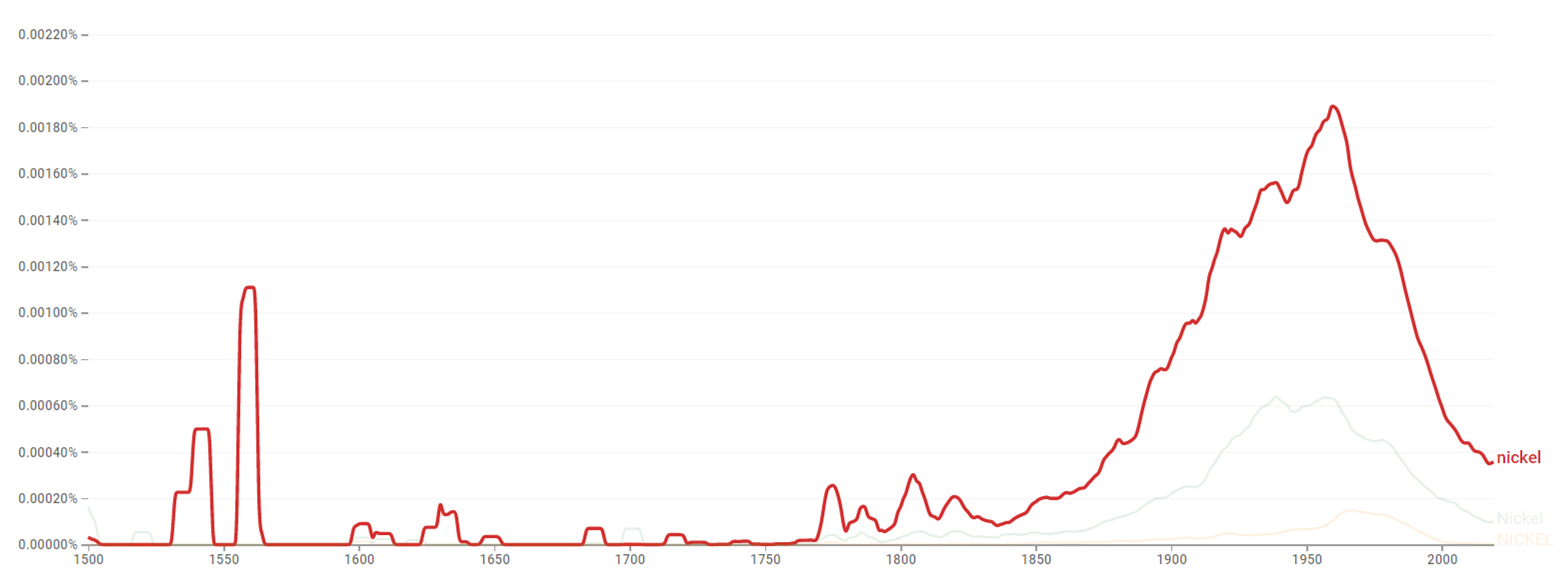
| + | The chart below shows {{w|Google Ngram Viewer}} data for Nickel, from 1500 to 2019.<ref>{{cite web |title=Nickel |url=https://books.google.com/ngrams/graph?content=Nickel&year_start=1500&year_end=2019&corpus=26&smoothing=3&case_insensitive=true |website=books.google.com |access-date=22 March 2021 |language=en}}</ref> | ||
| + | |||
| + | [[File:Nickel ngram.png|thumb|center|700px]] | ||
| + | |||
| + | === Wikipedia Views === | ||
| + | |||
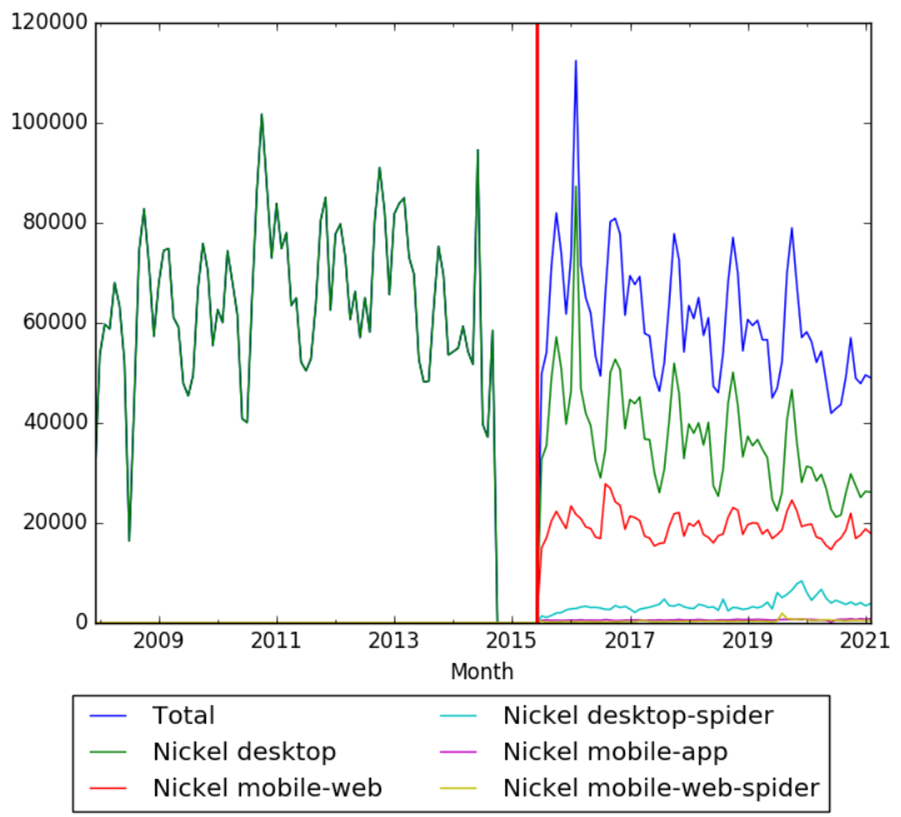
| + | The chart below shows pageviews of the English Wikipedia article {{w|Nickel}}, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to February 2021.<ref>{{cite web |title=Nickel |url=https://wikipediaviews.org/displayviewsformultiplemonths.php?page=Nickel&allmonths=allmonths&language=en&drilldown=all |website=wikipediaviews.org |access-date=22 March 2021}}</ref> | ||
| + | |||
| + | [[File:Nickel wv.png|thumb|center|450px]] | ||
==Meta information on the timeline== | ==Meta information on the timeline== | ||
| Line 92: | Line 208: | ||
===How the timeline was built=== | ===How the timeline was built=== | ||
| − | The initial version of the timeline was written by [[User: | + | The initial version of the timeline was written by [[User:Sebastian]]. |
{{funding info}} is available. | {{funding info}} is available. | ||
| Line 104: | Line 220: | ||
===What the timeline is still missing=== | ===What the timeline is still missing=== | ||
| − | + | * [https://resourceworld.com/giga-metals-responds-to-elon-musks-call-to-action-for-nickel-miners/] | |
===Timeline update strategy=== | ===Timeline update strategy=== | ||
Latest revision as of 21:00, 21 March 2024
This is a timeline of nickel. One of the most widely used metals on the planet, nickel is used in over 300,000 different products. It is most often found in steels and metal alloys. It is also used in the production of batteries and permanent magnets.[1]
Contents
Sample questions
The following are some interesting questions that can be answered by reading this timeline:
- When is the nickel date of discovery?
- How was nickel discovered?
- Where was nickel first discovered?
Big picture
| Time period | Development summary |
|---|---|
| Ancient history | Nickel is already used industrially as an alloying metal almost 2,000 years before it is isolated and recognized as a new element.[2] Its production sees an early development in China. |
| 15th century | German miners, who believe they can extract copper from nickel ores in Saxony, refer to the metal as kupfernickel ("the devil's copper"), partly due to their futile attempts to extract copper from the ore, but also likely in part due to the health effects caused by the high arsenic content in the ore.[1] |
| 18th century | Nickel is discovered in Sweden in this century, by Axel Fredrik Cronstedt. |
| 19th century | Nickel's use in plating and alloys with copper and zinc begins.[3] Since the mid-century, nickel is the component of coins in the United States, India, Switzerland, and Canada.<?> |
| 20th century | Early in the century, the discovery of large scale deposits in Russia and South Africa makes large-scale production of nickel possible. A significant increase in demand of the metal results from World War I and World War II.[1] Along with development in society, the nickel industry develops rapidly since 1974, and the metal plays an important role in the stainless steel industry, battery industry and certain other industries. Nickel consumption increases greatly between 1974 and 2011.[4] |
| 21st century | More than 1.4 million tons of primary or new nickel is produced worldwide.[5] As of 2010, the largest producers of nickel are Russia, Canada, Australia, and Indonesia. The largest producers of refined nickel are Norilsk Nickel, Vale S.A., and Jinchuan Group Ltd. Only a small percentage of nickel is produced from recycled materials,[1] however, nickel recycling industry is developing fast.[4] Nickel is now recognized as one of the most valuable mined materials, making nickel mining an important industry.[5] |
Full timeline
| Year | Event type | Details | Country/region |
|---|---|---|---|
| 3500 BC | Application | Syrian bronzes contain a small amount of nickel.[4] | Syria |
| 1700 BC – 1400 BC | Application | Chinese manuscripts suggest use of white copper (cupronickel).[4] | China |
| 1500 BC | Application | Chinese documents from that time make reference to "white copper" (baitong), whis is very likely an alloy of nickel and silver.[1] | China |
| 235 BC | Application | Coins in China are made of nickel.[4][5][6][7] | China |
| 200 BC | Application | The Chinese make substantial amounts of a white alloy from zinc and copper-nickel ore found in Yunnan province. The alloy, known as pai-t'ung, is exported to the Middle East and even to Europe.[2] | China |
| 1751 | Scientific development | Swedish chemist Axel Fredrik Cronstedt first extracts pure nickel from the mineral niccolite (NiAs).[1][3][7] Expecting to extract copper from this mineral, Cronsted obtains instead a white metal that he ends up calling nickel after the mineral from which it was extracted.[8] | Sweden |
| 1775 | Scientific development | Swedish mineralogist Torbern Bergman first fully purifies nickel.[9][10][11] | |
| 1776 | Application | It is established that the Chinese alloy pai-t'ung, now called nickel-silver, is composed of copper, nickel, and zinc.[2] | |
| 1796 | Scientific development | English chemist William Nicholson publishes First Principles of Chemistry. About nickel, Nicholson writes:
This metallic substance has not been applied to any use; and the chief attention of those chemists who have examined it has been directed to obtain it in a state of purity; which however has not been accomplished... Nickel has been thought to be a modification of iron... So long as no one is able to produce this metal from pure iron or copper, and to explain in an intelligible way the process by which it can be generated, we must continue to regard it as a peculiar substance, possessing distinct properties. The general opinions of chemists concur in admitting the force of this reasoning.[12] |
United Kingdom |
| 1820 | Application | British chemist and natural philosopher Michael Faraday carries out several experiments in making nickel-containing steels, but fails to interest the steelmakers.[7] | United Kingdom |
| 1837 | Application | G. Bird describes electrodeposition of nickel chloride or sulphate on platinum. This process results in a thin crust of nickel on the platinum substrate.[13][14][15] | |
| 1840 | Application | Joseph Shore applies for a patent for nickel plating.[16] | |
| 1842 | Application | Böttger in Frankfurt succeeds in depositing nickel from a solution of nickel ammonium sulfate.[16] | Germany |
| 1844 | Production | Demand of nickel-silver is stimulated in England by the development of silver electroplating, for which it is found to be the most desirable base. The use of pure nickel as a corrosion-resistant electroplated coating would develop a little later.[2] | United Kingdom |
| 1860 | Application | An alloy of 25% nickel and 75% copper, essentially white in color, is adopted for coinage in Belgium.[2] | Belgium |
| 1865 | Application | An alloy copper-nickel is adopted for coinage in the United States.[2] | United States |
| 1865 | Reserve | Big deposits of nickel ores are discovered in New Caledonia.[7] | New Caledonia |
| 1868 | Application | W.H. Remington from Boston commences the deposition of nickel on a commercial scale using a nickel ammonium chloride solution.[16][17] | United States |
| 1869 | Application | Dr I. Adams in the United States patents a nickel ammonium sulphate bath.[18][13][16][17][19][20] | United States |
| 1877 | Production | Production of nickel starts in New Caledonia in the South Pacific.[2][21] | New Caledonia |
| 1878 | Application | Weston obtains a patent for the addition of boric acid to nickel plating solutions.[16] | |
| 1880 | Application | Nickel plating salts are being made in Vienna by Pfanhauser and in Birmingham by Canning.[17] | Austria, United Kingdom |
| 1881 | Application | Coins of nearly pure nickel are first used in Switzerland.[4][2] | Switzerland |
| 1889 | Application | British engineer James Riley makes a presentation to the Iron and Steel Institute of Great Britain on how the introduction of nickel could strenghten traditional steels. Riley's presentation results in a growing awareness of nickel's beneficial alloying properties and coincides with the discovery of large nickel deposits in New Caledonia and Canada.[1][22][23] | United Kingdom |
| 1890 | Scientific development | Nickel tetracarbonyl Ni(CO)4, is discovered. This compound, in which nickel exhibits a zero oxidation state, is used primarily as a carrier of carbon monoxide in the synthesis of acrylates (compounds used in the manufacture of plastics) from acetylene and alcohols. Nickel carbonyl is the first of a class of compounds called metal carbonyls to be discovered.[24] | |
| 1890 | Scientific development | A paper entitled Action of Carbon Monoxide on Nickel, by Ludwig Mond, Carl Langer and Friedrich Qpincke, describes the formation of a gaseous compound of nickel and carbon monoxide by the reaction of the latter with finely divided nickel.[23] | United Kingdom |
| 1898 | Application | Invar, an alloy containing 36% nickel, is discovered. With the balance iron, invar is notable for its extremely small thermal expansion. Along with later-developed nickel alloys, invar would have many applications ranging from thermostats to balance wheels for watches, metal-to-glass seals essential to electric lights, and radio tubes.[2][25][26] | |
| 1899 | Technology | W. Browne, of the Canadian Copper Co., develops an electrolytic process for the separation of nickel and copper.[23] | Canada |
| 1899 | Technology | German-born British chemist Ludwig Mond develops the now called Mond Process, for extraction and purification of nickel.[5] | United Kingdom |
| 1901 | Technology | Thomas Edison is awarded a patent por nickel-zinc (NiZn) chemistry. However, Edison would never be able to commercialize NiZn products. NiZn electrochemistry is found to have great potential because of its high energy density, high efficiency rating and temperature tolerance advantages compared to other battery chemistries.[27] | United States |
| 1905 | Reserve | The Copper Cliff-Sudbury, Ontario region in Canada becomes the world's largest source of nickel.[2] | Canada |
| 1915 | Scientific development | An investigation by H.T. Kalmus and coworkers dealing with cobalt as well as nickel is summarized in the publication Transactions of the Electrochemical Society. This work leads the authors to the opinion that nickel could not be plated under commercial conditions at current densities as high as those possible for cobalt.[19] | |
| 1916 | Application | O.P. Watts develops a rapid nickel plating bath.[16] | |
| 1918 | Production | The refining of nickel to the finished metal product begins in Canada with the building of Inco's refinery at Port Colborne, Ontario, using hydroelectric power from Niagara Falls.[28] | Canada |
| 1921 | Technology | The use of pH measurements for controlling nickel baths is first suggested. By the end of the decade, it would become a common industrial practice.[17] | |
| 1924 | Application | Nickel is used to plate automobile bumpers.[19] | |
| 1930 – 1935 | Application | Nickel-containing permanent-magnet alloys are developed in Japan.[2] | Japan |
| 1942 | Technology | W.A. Wesley and E.J. Roehl describe a bath of nickel sulfate, ammonium chloride and boric acid that could be operated at high pH to produce deposits with hardness in the range of 350-500 Vickers and tensile strength around 150,000 psi.[19] | |
| 1945 – 1955 | Application | Research by the International Nickel Company, Inc. (Inco) demonstrates the efficacy of multilayer coatings in enhancing corrosion protection of plated nickel.[19] | |
| 1959 | Technology | George Gutzeit, one of the inventors of the Kanigen process, presents a comprehensive discussion of the chemistry of the catalytic nickel process.[19][29] | |
| 1970 – 1975 | Application | The nickel-hydrogen battery is proposed as the leading candidate for rechargeable aerospace batteries.[30] | |
| c.1979 | Production | Nickel production in Soviet Russia surpasses that in Canada.[2] | Russia |
| 1980 | Application | Falconbridge Nickel Co. and Inco introduce round forms of pure electrolytic nickel for plating.[19] | |
| 1993 | Technology | Stanford University chemist Morris Eisenberg creates a new electrolyte that enables a significant increase in the NiZn battery cycle life.[27] | United States |
| 1998 (December) | Financial | Nickel trades at a record low of 3730.50.[31] | |
| 2007 (May) | Financial | Nickel trades at an all time high of 54050.[31] | |
| 2010 | Production | As of date, the largest producers of nickel are Russia, Canada, Australia, and Indonesia.[1] |
Numerical and visual data
Google Scholar
The table below summarizes per-year mentions on Google Scholar as of May 17, 2021.
| Year | nickel | nickel research | nickel properties | nickel applications | nickel technology |
|---|---|---|---|---|---|
| 1980 | 22,100 | 10,100 | 9,640 | 4,610 | 7,010 |
| 1985 | 23,600 | 11,700 | 10,300 | 5,070 | 6,840 |
| 1990 | 30,300 | 15,400 | 13,800 | 7,490 | 9,370 |
| 1995 | 45,300 | 22,500 | 18,300 | 11,300 | 13,900 |
| 2000 | 83,700 | 39,600 | 28,800 | 16,400 | 19,900 |
| 2002 | 96,800 | 49,400 | 33,600 | 18,900 | 22,900 |
| 2004 | 120,000 | 63,600 | 44,600 | 25,300 | 32,000 |
| 2006 | 141,000 | 80,500 | 55,600 | 33,300 | 40,700 |
| 2008 | 151,000 | 92,600 | 66,100 | 40,900 | 48,100 |
| 2010 | 159,000 | 115,000 | 77,700 | 52,700 | 58,900 |
| 2012 | 199,000 | 143,000 | 102,000 | 71,800 | 79,200 |
| 2014 | 194,000 | 145,000 | 109,000 | 87,200 | 87,000 |
| 2016 | 195,000 | 145,000 | 113,000 | 91,100 | 89,600 |
| 2017 | 178,000 | 134,000 | 113,000 | 92,000 | 93,800 |
| 2018 | 140,000 | 121,000 | 97,700 | 86,800 | 84,700 |
| 2019 | 109,000 | 85,800 | 78,600 | 71,800 | 72,100 |
| 2020 | 67,900 | 52,800 | 59,600 | 48,800 | 44,800 |
Google Trends
The comparative chart below shows Google Trends data for Nickel (Chemical element), Stainless steel (Search Term) and Chrome plating (Topic), from January 2004 to March 2021, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[32]
Google Ngram Viewer
The chart below shows Google Ngram Viewer data for Nickel, from 1500 to 2019.[33]
Wikipedia Views
The chart below shows pageviews of the English Wikipedia article Nickel, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to February 2021.[34]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by User:Sebastian.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
Timeline update strategy
See also
External links
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Metal Profile: Nickel". thebalance.com. Retrieved 9 August 2018.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "Nickel processing". britannica.com. Retrieved 9 August 2018.
- ↑ 3.0 3.1 "THE HISTORY OF NICKEL". twin-metals.com. Retrieved 9 August 2018.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Nickel: discovery and industry development". metalpedia.asianmetal.com. Retrieved 9 August 2018.
- ↑ 5.0 5.1 5.2 5.3 "NICKEL MINING AND PROCESSING: EVERYTHING YOU NEED TO KNOW". generalkinematics.com. Retrieved 25 August 2018.
- ↑ "Ni - Nickel". eperiodictable.com. Retrieved 25 August 2018.
- ↑ 7.0 7.1 7.2 7.3 "Metal Story: Nickel (Ni)". oristarcorp.com. Retrieved 25 August 2018.
- ↑ "Nickel: historical information". webelements.com. Retrieved 30 November 2019.
- ↑ Holleman, A. F.; Wiberg, Egon; Wiberg, Nils. Inorganic Chemistry.
- ↑ "What in the Word?! The bedeviling origins of 'nickel'". blog.oxforddictionaries.com. Retrieved 25 August 2018.
- ↑ Morris, Richard. The Last Sorcerers: The Path from Alchemy to the Periodic Table.
- ↑ Enghag, Per. Encyclopedia of the Elements: Technical Data - History - Processing - Applications.
- ↑ 13.0 13.1 "The History of Nickel Plating". thomasnet.com. Retrieved 9 August 2018.
- ↑ Dennis, J. K.; Such, T. E. Nickel and Chromium Plating.
- ↑ Wang, J; Gabe, D.R. "The chemistry of nickel electroplating solutions".
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 "Nickel Plating Primer". pfonline.com. Retrieved 25 August 2018.
- ↑ 17.0 17.1 17.2 17.3 Dennis, J. K.; Such, T. E. Nickel and Chromium Plating.
- ↑ Popular Science Aug 1884.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 "A Retrospective View of Nickel Plating". pfonline.com. Retrieved 25 August 2018.
- ↑ "Week 31". acs.org. Retrieved 25 August 2018.
- ↑ "Nickel - Mining and metallurgy". croixdusud.info. Retrieved 25 August 2018.
- ↑ Sparrow, Giles. Nickel.
- ↑ 23.0 23.1 23.2 Dennis, W.H. Metallurgy: 1863-1963.
- ↑ "Nickel". britannica.com. Retrieved 9 August 2018.
- ↑ Instruments & Control Systems, Volume 13. Chilton Company, 1940.
- ↑ "Invar". britannica.com. Retrieved 25 August 2018.
- ↑ 27.0 27.1 "History". zincfive.com. Retrieved 9 August 2018.
- ↑ "Nickel". uwaterloo.ca. Retrieved 25 August 2018.
- ↑ "Historical Highlights of Electroless Plating". pfonline.com. Retrieved 25 August 2018.
- ↑ "A History of Nickel Hydrogen Batteries" (PDF). electrochem.org. Retrieved 9 August 2018.
- ↑ 31.0 31.1 "Nickel". tradingeconomics.com. Retrieved 9 August 2018.
- ↑ "Nickel, Stainless steel and Chrome plating". Google Trends. Retrieved 22 March 2021.
- ↑ "Nickel". books.google.com. Retrieved 22 March 2021.
- ↑ "Nickel". wikipediaviews.org. Retrieved 22 March 2021.