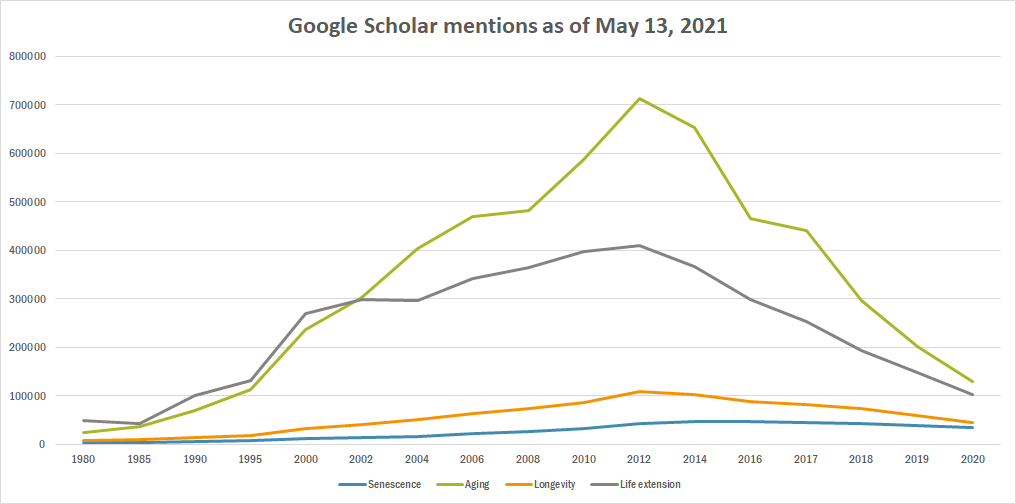
Difference between revisions of "Timeline of senescence research"
| (99 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Attribute English Wikipedia|original-exists=yes}} | {{Attribute English Wikipedia|original-exists=yes}} | ||
| − | This page is a '''timeline of [[wikipedia:senescence|senescence]] research''', including major theories, breakthroughs and organizations. | + | This page is a '''timeline of [[wikipedia:senescence|senescence]] research''', including major theories, breakthroughs and organizations. Senescence here is defined as "the process of physiological or biological decay leading to increasing mortality rates and/or decreasing fertility rates with age". This should be distinguished from "aging", which can be viewed simply as the march of time, with no physiological decay implied. |
== Sample questions == | == Sample questions == | ||
| Line 9: | Line 9: | ||
* What are some of the numerous theories of aging having been proposed throughout history? | * What are some of the numerous theories of aging having been proposed throughout history? | ||
** Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Theory". | ** Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Theory". | ||
| + | ** You will mostly see the description of a selected number among hundreds of theories of aging, including the most relevant. | ||
* What are some significant studies on senescence conducted in research labs? | * What are some significant studies on senescence conducted in research labs? | ||
| − | ** Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Laboratory". | + | ** Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Laboratory research". |
| + | ** You will see some notable lab discoveries related to the field of senescence. | ||
| + | * What are some other types of research mentioned in this timeline? | ||
| + | ** Sort the full timeline by "Research type (when applicable)". | ||
| + | ** Other types of research mentioned are: Clinical study, Concept development, and {{w|Data science}}. | ||
| + | * What are some organizations established with the purpose to promote the scientific development in the field of senescence? | ||
| + | ** Sort the full timeline by "Event type" and look for the group of rows with value "Organization". | ||
| + | ** You will see some non-profit organizations, other private, as well as some important governmental organizations focused on both senescence and aging. | ||
* What are some notable publications specialized in the field of senescence? | * What are some notable publications specialized in the field of senescence? | ||
** Sort the full timeline by "Event type" and look for the group of rows with value "Literature". | ** Sort the full timeline by "Event type" and look for the group of rows with value "Literature". | ||
| Line 18: | Line 26: | ||
** Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Concept introduction". | ** Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Concept introduction". | ||
** You will see descriptions around concepts like gerontology, senescence, and programmed cell-death. | ** You will see descriptions around concepts like gerontology, senescence, and programmed cell-death. | ||
| + | * Other events are described under the following types: "Conference", "Policy", and "Recognition". | ||
==Big picture== | ==Big picture== | ||
| Line 29: | Line 38: | ||
|Renaissance–18th century || Some themes around which aging and senescence research revolve are the idea that senescence is itself an illness, the image of the aged body as a lamp in which life-fuel has run out, the character alterations of elders, and the attempt to prolong life through specific diet or by substituting damaged body parts.<ref name=history-of-research/> | |Renaissance–18th century || Some themes around which aging and senescence research revolve are the idea that senescence is itself an illness, the image of the aged body as a lamp in which life-fuel has run out, the character alterations of elders, and the attempt to prolong life through specific diet or by substituting damaged body parts.<ref name=history-of-research/> | ||
|- | |- | ||
| − | | | + | | 19th century || The modern evolutionary theory of senescence is rooted in the late century.<ref name="Shefferson">{{cite book |last1=Shefferson |first1=Richard P. |last2=Jones |first2=Owen R. |last3=Salguero-Gómez |first3=Roberto |title=The Evolution of Senescence in the Tree of Life |date=23 February 2017 |publisher=Cambridge University Press |isbn=978-1-107-07850-5 |url=https://books.google.com.ar/books/about/The_Evolution_of_Senescence_in_the_Tree.html?id=8TQEDgAAQBAJ&source=kp_book_description&redir_esc=y |language=en}}</ref> Starting from the so-called "{{w|fin-de-siècle}} period, scientific optimism flourishes, and life-extensionism represents the most radical form of the trend.<ref name=ilia-stambler>{{cite book |date=2014 |title=A History of Life-Extensionism In The Twentieth Century |url= |location=Rison Lezion, Israel |publisher=Longevity History |page= |isbn=1500818577}}</ref> Life expectancy starts to rise in the Western world.<ref>{{cite web |url=https://ourworldindata.org/life-expectancy/ |title=Life Expectancy}}</ref> |
|- | |- | ||
| − | | 20th century || Since the 1930s, it is understood that restricting calories can extend lifespan in laboratory animals.<ref name="kunlin"/> After the Second World War, modern knowledge accumulates. The 1950s see the first attempts to distinguish, measure and compare the functional and chronological ages of individuals, of which biological considerations were an important component.<ref name="Anti-agi">{{cite journal |last1=Fletcher |first1=James Rupert |title=Anti-aging technoscience & the biologization of cumulative inequality: Affinities in the biopolitics of successful aging |journal=Journal of Aging Studies |date=December 2020 |volume=55 |pages=100899 |doi=10.1016/j.jaging.2020.100899 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7576313/ |issn=0890-4065}}</ref><ref>{{cite journal |last1=Binstock |first1=Robert H. |title=The War on “Anti-Aging Medicine” |journal=The Gerontologist |date=February 2003 |volume=43 |issue=1 |pages=4–14 |doi=10.1093/geront/43.1.4}}</ref> The beginning of the term “senescence” in the context of mammalian cell cultures begins in the 1960s with the work of [[w:Leonard Hayflick|Hayflick]] and Moorhead.<ref name="Ogrodnik"/> Also by this same decade, biologists start becoming aware of mortality leveling-off.<ref name="Handbook of the">{{cite book |title=Handbook of the biology of aging |date=2006 |publisher=Elsevier Academic Press |location=Amsterdam |isbn=9780120883875 |edition=6th}}</ref> In the 1980s, the field of life-span psychology is born.<ref>{{cite journal |last1=Fried |first1=Linda P. |last2=Rowe |first2=John W. |title=Health in Aging — Past, Present, and Future |journal=New England Journal of Medicine |date=2020-10-01 |volume=383 |issue=14 |pages=1293–1296 |doi=10.1056/NEJMp2016814}}</ref> | + | | 20th century || Since the 1930s, it is understood that restricting calories can extend lifespan in laboratory animals.<ref name="kunlin"/> After the Second World War, modern knowledge accumulates. The 1950s see the first attempts to distinguish, measure and compare the functional and chronological ages of individuals, of which biological considerations were an important component.<ref name="Anti-agi">{{cite journal |last1=Fletcher |first1=James Rupert |title=Anti-aging technoscience & the biologization of cumulative inequality: Affinities in the biopolitics of successful aging |journal=Journal of Aging Studies |date=December 2020 |volume=55 |pages=100899 |doi=10.1016/j.jaging.2020.100899 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7576313/ |issn=0890-4065}}</ref><ref>{{cite journal |last1=Binstock |first1=Robert H. |title=The War on “Anti-Aging Medicine” |journal=The Gerontologist |date=February 2003 |volume=43 |issue=1 |pages=4–14 |doi=10.1093/geront/43.1.4}}</ref> The beginning of the term “senescence” in the context of mammalian cell cultures begins in the 1960s with the work of [[w:Leonard Hayflick|Hayflick]] and Moorhead.<ref name="Ogrodnik"/> Also by this same decade, biologists start becoming aware of mortality leveling-off.<ref name="Handbook of the">{{cite book |title=Handbook of the biology of aging |date=2006 |publisher=Elsevier Academic Press |location=Amsterdam |isbn=9780120883875 |edition=6th}}</ref> Around the start of the 1970s, senescence research starts broadening its evolutionary scope.<ref name="Shefferson"/> During the mid-1970s, there is considerable interest in the idea that cells might become destabilized and thereby precipitates onto a pathway of progressive deterioration.<ref name="Shefferson"/> In the 1980s, the field of life-span psychology is born.<ref>{{cite journal |last1=Fried |first1=Linda P. |last2=Rowe |first2=John W. |title=Health in Aging — Past, Present, and Future |journal=New England Journal of Medicine |date=2020-10-01 |volume=383 |issue=14 |pages=1293–1296 |doi=10.1056/NEJMp2016814}}</ref> In the 1990s, Caleb Finch gives serious consideration to organisms that exhibit negligible senescence and experience no, or only very small, increases in mortality rate with age.<ref name="Shefferson"/> |
|- | |- | ||
| − | | 21st century || Modern anti-aging organizations merge and their proliferation multiplies toward the 2000s.<ref name=ilia-stambler/> In the 21st century, research activity increases, as well as interdisciplinary collaboration of senescence research with other fields, such as {{w|data science}}. In the second half of 2010s official discussions about the possibility of recognizing aging as a disease | + | | 21st century || Modern anti-aging organizations merge and their proliferation multiplies toward the 2000s.<ref name=ilia-stambler/> In the 21st century, research activity increases, as well as interdisciplinary collaboration of senescence research with other fields, such as {{w|data science}}. In the second half of 2010s official discussions about the possibility of recognizing aging as a disease emerge.<ref>{{cite journal|last1=Zhavoronkov |first1=Alexander |last2=Bhupinder |first2=Bhullar |date=4 October 2015 |title=Classifying aging as a disease in the context of ICD-11 |url= |journal={{w|Frontiers in Genetics}} |volume=6 |issue= |pages=326 |doi=10.3389/fgene.2015.00326 |pmc=4631811 |pmid=26583032}}</ref><ref>{{cite journal|last1=Stambler |first1=Ilia |date=1 October 2017 |title=Recognizing Degenerative Aging as a Treatable Medical Condition: Methodology and Policy |url=http://www.aginganddisease.org/article/2017/2152-5250/ad-8-5-583.shtml |journal={{w|Aging and Disease}} |volume=8 |issue=5 |pages=583–589 |doi=10.14336/AD.2017.0130 |pmid=28966803|pmc=5614323 |doi-access=free }}</ref><ref>{{cite journal|date=1 August 2018 |title=Opening the door to treating ageing as a disease |url=https://www.thelancet.com/journals/landia/article/PIIS2213-8587(18)30214-6/fulltext |journal={{w|The Lancet Diabetes & Endocrinology}} |volume=6 |issue=8 |pages=587 |doi=10.1016/S2213-8587(18)30214-6|last1=<!---->}}</ref><ref>{{cite journal|last1=Calimport |first1=Stuart |last2=Bentley |first2=Barry L. |last3=Stewart |first3=Claire E. |last4=Pawelec |first4=Graham |last5=Scuteri |first5=Angelo |last6=Vinciguerra |first6=Manlio |last7=Slack |first7=Cathy |last8=Chen |first8=Danica |last9=Harries |first9=Lorna W. |last10=Marchant |first10=Gary |last11=Fleming |first11=G. Alexander |last12=Conboy |first12=Michael |last13=Antebi |first13=Adam |last14=Small |first14=Gary W. |last15=Gil |first15=Jesus |last16=Lakatta |first16=Edward G. |last17=Richardson |first17=Arlan |last18=Rosen |first18=Clifford |last19=Nikolich |first19=Karoly |last20=Wyss-Coray |first20=Tony |last21=Steinman |first21=Lawrence |last22=Montine |first22=Thomas |last23=Magalhães |first23=João Pedro de |last24=Campisi |first24=Judith |last25=Church |first25=George |display-authors=1 |date=1 October 2019 |title=To help aging populations, classify organismal senescence |url= |journal=[[w:Science (journal)|Science]] |volume=366 |issue=6465 |pages=576–578 |doi=10.1126/science.aay7319}}</ref><ref>{{cite journal|last1=Khaltourina |first1=Daria|last2=Matveyev |first2=Yuri |last3=Alekseev |first3=Aleksey |last4=Cortese |first4=Franco |last5=Ioviţă |first5=Anca |date=July 2020 |title=Aging Fits the Disease Criteria of the International Classification of Diseases |url=https://www.sciencedirect.com/science/article/abs/pii/S0047637420300257 |journal= Mechanisms of Ageing and Development|volume=189 |page=111230 |doi=10.1016/j.mad.2020.111230}}</ref> |
|- | |- | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Full timeline== | ==Full timeline== | ||
| Line 110: | Line 51: | ||
! Year/period !! Type of Event !! Research type (when applicable) || Event !! Location | ! Year/period !! Type of Event !! Research type (when applicable) || Event !! Location | ||
|- | |- | ||
| − | | c. 99 BC – c. 55 BC || Scientific development || || Roman poet and philosopher {{w|Lucretius}} argues that aging and death are beneficial because they make room for the next generation. This view will persist among biologists well into the 20th century.<ref name=the-evolution-of-aging>{{cite magazine |author1=Daniel Fabian |author2=Thomas Flatt |title=The Evolution of Aging |url=http://www.nature.com/scitable/knowledge/library/the-evolution-of-aging-23651151 |magazine=Nature |location= |publisher= |date= |access-date= }}</ref> || || [[File:Lucretius1.png|thumb|center|100px]] | + | | c. 99 BC – c. 55 BC || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Roman poet and philosopher {{w|Lucretius}} argues that aging and death are beneficial because they make room for the next generation. This view will persist among biologists well into the 20th century.<ref name=the-evolution-of-aging>{{cite magazine |author1=Daniel Fabian |author2=Thomas Flatt |title=The Evolution of Aging |url=http://www.nature.com/scitable/knowledge/library/the-evolution-of-aging-23651151 |magazine=Nature |location= |publisher= |date= |access-date= }}</ref> || || [[File:Lucretius1.png|thumb|center|100px|{{w|Lucretius}}]] |
|- | |- | ||
| − | |5th century || Scientific development || || Early formulations, described by | + | |5th century || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Early formulations, described by {{w|Hippocrates}}' system of four [[wikipedia:Humorism|humours]], theorize old age as a consequence of the gradual consumption of the innate heat with the inevitable loss of body moisture.<ref name=history-of-research/> || {{w|Greece}} || [[File:Hippocrates.jpg|thumb|center|100px|{{w|Hippocrates}}]] |
|- | |- | ||
| − | | 1825 || Scientific development || || British mathematician {{w|Benjamin Gompertz}} proposes an exponential increase in death rates with age, giving birth to what later will be called The [[wikipedia:Gompertz-Makeham law of mortality|Gompertz-Makeham law]].<ref>{{cite journal |last=Gompertz |first=B. |year=1825 |title=On the Nature of the Function Expressive of the Law of Human Mortality, and on a New Mode of Determining the Value of Life Contingencies |journal=Philosophical Transactions of the Royal Society |volume=115 |issue= |pages=513–585 |id= |url=http://visualiseur.bnf.fr/Visualiseur?Destination=Gallica&O=NUMM-55920 |doi=10.1098/rstl.1825.0026}} | + | | 1825 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || British mathematician {{w|Benjamin Gompertz}} proposes an exponential increase in death rates with age, giving birth to what later will be called The [[wikipedia:Gompertz-Makeham law of mortality|Gompertz-Makeham law]].<ref>{{cite journal |last=Gompertz |first=B. |year=1825 |title=On the Nature of the Function Expressive of the Law of Human Mortality, and on a New Mode of Determining the Value of Life Contingencies |journal=Philosophical Transactions of the Royal Society |volume=115 |issue= |pages=513–585 |id= |url=http://visualiseur.bnf.fr/Visualiseur?Destination=Gallica&O=NUMM-55920 |doi=10.1098/rstl.1825.0026}} |
| − | </ref><ref name="Leonid">Leonid A. Gavrilov & Natalia S. Gavrilova (1991) The Biology of Life Span: A Quantitative Approach. New York: Harwood Academic Publisher, ISBN 3-7186-4983-7</ref> The simplest form of the law is: '''p = a + b<sup>x</sup>'''. According to the law, the probability of death '''p''' is defined as the sum of age-independent component '''a''' and the component depending of age '''b<sup>x</sup>''' which with age increases exponentially. If we place organisms in an absolutely protected environment and in this way make the first component negligible, the probability of death will be completely defined by the second component which actually describes the probability to die from aging. || {{w|United Kingdom}} || [[File:Gompertz.png|thumb|center|110px]] | + | </ref><ref name="Leonid">Leonid A. Gavrilov & Natalia S. Gavrilova (1991) The Biology of Life Span: A Quantitative Approach. New York: Harwood Academic Publisher, ISBN 3-7186-4983-7</ref> The simplest form of the law is: '''p = a + b<sup>x</sup>'''. According to the law, the probability of death '''p''' is defined as the sum of age-independent component '''a''' and the component depending of age '''b<sup>x</sup>''' which with age increases exponentially. If we place organisms in an absolutely protected environment and in this way make the first component negligible, the probability of death will be completely defined by the second component which actually describes the probability to die from aging. || {{w|United Kingdom}} || [[File:Gompertz.png|thumb|center|110px|{{w|Benjamin Gompertz}}]] |
|- | |- | ||
| − | | 1882 || Scientific development ||[[w:Category:Theories of biological aging|Theory]] || German evolutionary biologist {{w|August Weismann}} introduces the wear and tear theory of aging, which sustains that cells and tissues have vital parts that wear out resulting in aging. "Like components of an aging car, parts of the body eventually wear out from repeated use, killing them and then the body."<ref name="kunlin"/> || {{w|Germany}} | + | | 1882 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || German evolutionary biologist {{w|August Weismann}} introduces the wear and tear theory of aging, which sustains that cells and tissues have vital parts that wear out resulting in aging. "Like components of an aging car, parts of the body eventually wear out from repeated use, killing them and then the body."<ref name="kunlin"/> Aging would result from an accumulation of damage to cells, tissues, and organs in the body caused by dietary toxins and environmental agents, leading to the weakening and eventual death of the cells, tissues, and organs.<ref>{{cite web |title=APA Dictionary of Psychology |url=https://dictionary.apa.org/wear-and-tear-theory-of-aging |website=dictionary.apa.org |access-date=23 December 2021 |language=en}}</ref> || {{w|Germany}} |
|- | |- | ||
| − | | 1891 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || {{w|August Weismann}} proposes the first formal programmed aging theory as an evolutionary explanation of aging driven by group selection. His argument is that aging evolved to the advantage of the species (e.g., by replacing worn out individuals with younger ones), not the individual.<ref>{{cite web|url=http://www.programmed-aging.org/programmed_aging_theory_FAQ.html|title=Biological Aging Theory - Frequently asked Questions and Answers}}</ref><ref>{{cite web |title=A Weismann| URL=http://www.senescence.info/evolution_of_aging.html}}</ref> || {{w|Germany}} || [[File:August Weismann.jpg|thumb|center|100px]] | + | | 1891 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || German evolutionary biologist {{w|August Weismann}} proposes the first formal programmed aging theory as an evolutionary explanation of aging driven by group selection. His argument is that aging evolved to the advantage of the species (e.g., by replacing worn out individuals with younger ones), not the individual.<ref>{{cite web|url=http://www.programmed-aging.org/programmed_aging_theory_FAQ.html|title=Biological Aging Theory - Frequently asked Questions and Answers}}</ref><ref>{{cite web |title=A Weismann| URL=http://www.senescence.info/evolution_of_aging.html}}</ref> || {{w|Germany}} || [[File:August Weismann.jpg|thumb|center|100px|{{w|August Weismann}}]] |
|- | |- | ||
| 1903 || Scientific development || Concept introduction || Russian zoologist {{w|Ilya Mechnikov}} coins the term "{{w|gerontology}}".<ref>{{cite book | vauthors = Harris DK |title=Dictionary of Gerontology |url=https://archive.org/details/dictionaryofgero0000harr |url-access=registration |publisher=Greenwood Press |location=New York |year=1988 |page=[https://archive.org/details/dictionaryofgero0000harr/page/80 80]|isbn=9780313252877 }}</ref><ref>{{cite book| vauthors = Metchnikoff E|translator-last=Mitchell |translator-first=P Chalmers |year=1903 |title=The Nature of Man: Studies in Optimistic Philosophy |url=https://www.worldcat.org/title/nature-of-man-studies-in-optimistic-philosophy/oclc/173625 |language=en |location=New York and London |publisher=G.P. Putnam's Sons |oclc=173625}}</ref><ref name="grignolio">{{cite book| vauthors = Grignolio A, Franceschi C |title=eLS |date=15 June 2012 |chapter=History of Research into Ageing/Senescence |publisher=American Cancer Society |chapter-url=https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0023955 |doi=10.1002/9780470015902.a0023955|isbn=978-0470016176 }}</ref> || | | 1903 || Scientific development || Concept introduction || Russian zoologist {{w|Ilya Mechnikov}} coins the term "{{w|gerontology}}".<ref>{{cite book | vauthors = Harris DK |title=Dictionary of Gerontology |url=https://archive.org/details/dictionaryofgero0000harr |url-access=registration |publisher=Greenwood Press |location=New York |year=1988 |page=[https://archive.org/details/dictionaryofgero0000harr/page/80 80]|isbn=9780313252877 }}</ref><ref>{{cite book| vauthors = Metchnikoff E|translator-last=Mitchell |translator-first=P Chalmers |year=1903 |title=The Nature of Man: Studies in Optimistic Philosophy |url=https://www.worldcat.org/title/nature-of-man-studies-in-optimistic-philosophy/oclc/173625 |language=en |location=New York and London |publisher=G.P. Putnam's Sons |oclc=173625}}</ref><ref name="grignolio">{{cite book| vauthors = Grignolio A, Franceschi C |title=eLS |date=15 June 2012 |chapter=History of Research into Ageing/Senescence |publisher=American Cancer Society |chapter-url=https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0023955 |doi=10.1002/9780470015902.a0023955|isbn=978-0470016176 }}</ref> || | ||
| Line 125: | Line 66: | ||
| 1904 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Metchnikoff introduces the Autointoxication theory of aging, which is considered important at the whole animal level.<ref name="Dazhong"/> This is one of the first toxic theories as Metchnikoffs proposes the idea of autointoxication by products of bacterial origin, primarily from bacterial processes in the large intestine. Metchnikoff believes that even simple changes of acidity by the consumption of yogurt could inhibit the production of bacterial toxins.<ref name="Medvedev"/> || | | 1904 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Metchnikoff introduces the Autointoxication theory of aging, which is considered important at the whole animal level.<ref name="Dazhong"/> This is one of the first toxic theories as Metchnikoffs proposes the idea of autointoxication by products of bacterial origin, primarily from bacterial processes in the large intestine. Metchnikoff believes that even simple changes of acidity by the consumption of yogurt could inhibit the production of bacterial toxins.<ref name="Medvedev"/> || | ||
|- | |- | ||
| − | | 1908 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || German physiologist {{w|Max Rubner}} describes his {{w|rate-of-living theory}}, which proposes that a slow metabolism increases an animal's longevity. It states that fast basal metabolic rate corresponds to short maximum life span.<ref>{{cite journal |author1=Michael Ristow |author2=Kathrin Schmeisser |date= |title=Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS) |journal= Dose Response|publisher= |volume= 12|issue= |pages= 288–341|doi= 10.2203/dose-response.13-035.Ristow|pmc=4036400 |pmid= 24910588 |year=2014}}</ref><ref>Rubner, M. (1908). Das Problem det Lebensdaur und seiner beziehunger zum Wachstum und Ernarnhung. Munich: Oldenberg.</ref> Some studies conclude that the rate‐of‐living theory does not hold true for terrestrial vertebrates, and suggest that life expectancy is driven by selection arising from extrinsic mortality factors.<ref>{{cite journal |last1=Stark |first1=Gavin |last2=Pincheira‐Donoso |first2=Daniel |last3=Meiri |first3=Shai |title=No evidence for the ‘rate‐of‐living’ theory across the tetrapod tree of life |journal=Global Ecology and Biogeography |date=May 2020 |volume=29 |issue=5 |pages=857–884 |doi=10.1111/geb.13069}}</ref> || {{w|Germany}} || [[File:Max Rubner.jpg|thumb|center|100px]] | + | | 1908 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || German physiologist {{w|Max Rubner}} describes his {{w|rate-of-living theory}}, which proposes that a slow metabolism increases an animal's longevity. It states that fast basal metabolic rate corresponds to short maximum life span.<ref>{{cite journal |author1=Michael Ristow |author2=Kathrin Schmeisser |date= |title=Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS) |journal= Dose Response|publisher= |volume= 12|issue= |pages= 288–341|doi= 10.2203/dose-response.13-035.Ristow|pmc=4036400 |pmid= 24910588 |year=2014}}</ref><ref>Rubner, M. (1908). Das Problem det Lebensdaur und seiner beziehunger zum Wachstum und Ernarnhung. Munich: Oldenberg.</ref> Some studies conclude that the rate‐of‐living theory does not hold true for terrestrial vertebrates, and suggest that life expectancy is driven by selection arising from extrinsic mortality factors.<ref>{{cite journal |last1=Stark |first1=Gavin |last2=Pincheira‐Donoso |first2=Daniel |last3=Meiri |first3=Shai |title=No evidence for the ‘rate‐of‐living’ theory across the tetrapod tree of life |journal=Global Ecology and Biogeography |date=May 2020 |volume=29 |issue=5 |pages=857–884 |doi=10.1111/geb.13069}}</ref> Support for this theory is bolstered by studies linking a lower {{w|basal metabolic rate}} (evident with a lowered heartbeat) to increased life expectancy.<ref>{{cite web |last1=Hulbert |first1=A. J. |last2=Pamplona |first2=Reinald |last3=Buffenstein |first3=Rochelle |last4=Buttemer |first4=W. A. |title=Life and Death: Metabolic Rate, Membrane Composition, and Life Span of Animals |url=http://physrev.physiology.org/content/87/4/1175.full |website=Physiological Reviews |access-date=7 October 2021 |pages=1175–1213 |doi=10.1152/physrev.00047.2006 |date=1 October 2007}}</ref><ref>{{cite web |last1=Olshansky |first1=S. J. |last2=Rattan |first2=Suresh IS |title=What Determines Longevity: Metabolic Rate or Stability? |url=http://www.discoverymedicine.com/S-J-Olshansky/2009/07/25/what-determines-longevity-metabolic-rate-or-stability |website=Discovery Medicine |pages=359–362 |language=en |date=25 July 2009}}</ref> However, in a 2007 analysis it would be shown that, when modern statistical methods for correcting for the effects of body size and phylogeny are employed, metabolic rate does not correlate with longevity in mammals or birds.<ref>{{cite journal |last1=Magalhães |first1=João Pedro de |last2=Costa |first2=Joana |last3=Church |first3=George M. |title=An Analysis of the Relationship Between Metabolism, Developmental Schedules, and Longevity Using Phylogenetic Independent Contrasts |journal=The Journals of Gerontology: Series A |date=1 February 2007 |volume=62 |issue=2 |pages=149–160 |doi=10.1093/gerona/62.2.149}}</ref> || {{w|Germany}} || [[File:Max Rubner.jpg|thumb|center|100px|{{w|Max Rubner}}]] |
|- | |- | ||
| − | | 1928 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || American biologist {{w|Raymond Pearl}} describes the ''rate of living hypothesis'' as an expansion of the earlier theory by {{w|Max Rubner}}. It states that organisms with a high metabolic rate have shorter lives.<ref name=oxidative-stress>{{cite book |author= David Costantini |title=Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology |url=https://books.google.com.ar/books?id=rTe8BAAAQBAJ&pg=PA306&dq=Rate+Of+Living+Hypothesis&hl=es&sa=X&ved=0ahUKEwjui-iU5tDMAhXJIJAKHe4lBSMQ6AEILDAC#v=onepage&q=Pearl&f=false |location= |publisher= |page=306 |date= |isbn=}}</ref> || {{w|United States}} || [[File:Dr. Raymond Pearl, Food. Adm. LCCN2016824384 (cropped).jpg|thumb|center|100px]] | + | | 1922 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Ukrainian pathophysiologist {{w|Aleksandr Bogomolets}} proposes that the deterioration of the connective tissues cause aging.<ref>{{cite web |title=Cross linking theory of aging |url=https://www.eos.org.eg/ckfinder/userfiles/files/dujotixajikuxanajinevutar.pdf}}</ref> || {{w|Ukraine}} ({{w|Soviet Union}}) || [[File:Александр Александрович Богомолец (cropped).jpg|thumb|center|100px|{{w|Aleksandr Bogomolets}}]] |
| + | |- | ||
| + | | 1928 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || American biologist {{w|Raymond Pearl}} describes the ''rate of living hypothesis'' as an expansion of the earlier theory by {{w|Max Rubner}}. It states that organisms with a high metabolic rate have shorter lives.<ref name=oxidative-stress>{{cite book |author= David Costantini |title=Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology |url=https://books.google.com.ar/books?id=rTe8BAAAQBAJ&pg=PA306&dq=Rate+Of+Living+Hypothesis&hl=es&sa=X&ved=0ahUKEwjui-iU5tDMAhXJIJAKHe4lBSMQ6AEILDAC#v=onepage&q=Pearl&f=false |location= |publisher= |page=306 |date= |isbn=}}</ref> Further studies would demonstrate that rats kept on restricted diets and in cold environments live longer and that these conditions have the appearance and behavior of younger animals.<ref>{{cite web |title=Theory 5: Rate of Living Theory {{!}} Biology of Aging |url=https://courses.lumenlearning.com/atd-herkimer-biologyofaging/chapter/theory-5-rate-of-living-theory/ |website=courses.lumenlearning.com |access-date=7 October 2021}}</ref> || {{w|United States}} || [[File:Dr. Raymond Pearl, Food. Adm. LCCN2016824384 (cropped).jpg|thumb|center|100px|{{w|Raymond Pearl}}]] | ||
|- | |- | ||
| 1930 || Literature (journal) || || The first world's journal about aging and longevity is established in Japan.It's titled ''Acta Gerontologica Japonica'' (''Yokufuen Chosa Kenkyu Kiyo'').<ref>{{cite book|last=Stambler |first=Ilia |date=29 August 2014 |chapter=reference No. 438 |chapter-url=http://www.longevityhistory.com/read-the-book-online/#_edn438 |title=A History of Life-Extensionism in the Twentieth Century |url=https://www.researchgate.net/publication/315526897 |series=Longevity History |pages=540 |isbn=978-1500818579}}</ref> || {{w|Japan}} | | 1930 || Literature (journal) || || The first world's journal about aging and longevity is established in Japan.It's titled ''Acta Gerontologica Japonica'' (''Yokufuen Chosa Kenkyu Kiyo'').<ref>{{cite book|last=Stambler |first=Ilia |date=29 August 2014 |chapter=reference No. 438 |chapter-url=http://www.longevityhistory.com/read-the-book-online/#_edn438 |title=A History of Life-Extensionism in the Twentieth Century |url=https://www.researchgate.net/publication/315526897 |series=Longevity History |pages=540 |isbn=978-1500818579}}</ref> || {{w|Japan}} | ||
|- | |- | ||
| − | | 1934 || Scientific development || Laboratory || Mary Crowell and Clive McCay at {{w|Cornell University}} discover that {{w|calorie restriction}} can extend lifespan twofold in rats.<ref>{{cite book |last= Fossel |first=Michael |date= |title=The Telomerase Revolution: The Enzyme That Holds the Key to Human Aging |url= |location= |publisher= |page= |isbn=}}</ref> || {{w|United States}} | + | | 1934 || Scientific development || Laboratory research || Mary Crowell and Clive McCay at {{w|Cornell University}} discover that {{w|calorie restriction}} can extend lifespan twofold in rats.<ref>{{cite book |last= Fossel |first=Michael |date= |title=The Telomerase Revolution: The Enzyme That Holds the Key to Human Aging |url= |location= |publisher= |page= |isbn=}}</ref> || {{w|United States}} |
|- | |- | ||
| 1938 || Organization || || The German Society for Aging Research (German: ''Deutsche Gesellschaft für Altersforschung'') is founded in {{w|Leipzig}} as the first specialized society dedicated to the study of aging. The founder, Max Bürger, also establishes the specialized journal ''Zeitschrift für Altersforschung'' , which becomes the third such journal in the world after the previously mentioned Japanese and Romanian journals.<ref>{{cite book|last=Stambler |first=Ilia |date=29 August 2014 |chapter=Institutionalization of gerontology – Max Bürger |chapter-url=http://www.longevityhistory.com/read-the-book-online/#_Toc328320008 |title=A History of Life-Extensionism in the Twentieth Century |url=https://www.researchgate.net/publication/315526897 |series=Longevity History |pages=540 |isbn=978-1500818579}}</ref> || {{w|Germany}} | | 1938 || Organization || || The German Society for Aging Research (German: ''Deutsche Gesellschaft für Altersforschung'') is founded in {{w|Leipzig}} as the first specialized society dedicated to the study of aging. The founder, Max Bürger, also establishes the specialized journal ''Zeitschrift für Altersforschung'' , which becomes the third such journal in the world after the previously mentioned Japanese and Romanian journals.<ref>{{cite book|last=Stambler |first=Ilia |date=29 August 2014 |chapter=Institutionalization of gerontology – Max Bürger |chapter-url=http://www.longevityhistory.com/read-the-book-online/#_Toc328320008 |title=A History of Life-Extensionism in the Twentieth Century |url=https://www.researchgate.net/publication/315526897 |series=Longevity History |pages=540 |isbn=978-1500818579}}</ref> || {{w|Germany}} | ||
| Line 141: | Line 84: | ||
| 1940 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Nagorny introduces a theory of aging based on age changes, which is described as the stabilization and inactivation (insolubilization) of intercellular structural proteins. Nagorny postulates that aging is linked to the accumulation of intercellular inactivated "metaplasmic" proteins due to the inability of enzymes to destroy them.<ref name="Medvedev"/> || || | | 1940 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Nagorny introduces a theory of aging based on age changes, which is described as the stabilization and inactivation (insolubilization) of intercellular structural proteins. Nagorny postulates that aging is linked to the accumulation of intercellular inactivated "metaplasmic" proteins due to the inability of enzymes to destroy them.<ref name="Medvedev"/> || || | ||
|- | |- | ||
| − | | 1942 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Johan Björkstein proposes the cross‐linkage theory of aging. According to it, aging results from the accumulation of intra‐ and intermolecular covalent bonds between molecules, termed “cross‐links.”<ref>{{cite journal |last1=Diggs |first1=Jessica |title=The Cross‐Linkage Theory of Aging |journal=Encyclopedia of Aging and Public Health |date=2008 |pages=250–252 |doi=10.1007/978-0-387-33754-8_112 |url= |publisher=Springer US |language=en}}</ref><ref name="kunlin"/> || | + | | 1942 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Johan Björkstein proposes the cross‐linkage theory of aging. According to it, aging results from the accumulation of intra‐ and intermolecular covalent bonds between molecules, termed “cross‐links.”<ref>{{cite journal |last1=Diggs |first1=Jessica |title=The Cross‐Linkage Theory of Aging |journal=Encyclopedia of Aging and Public Health |date=2008 |pages=250–252 |doi=10.1007/978-0-387-33754-8_112 |url= |publisher=Springer US |language=en}}</ref><ref name="kunlin"/> Recent studies show that cross-linking reactions are involved in the age related changes in the studied proteins.<ref>{{cite web |title=Theory 7: Cross-linkage Theory {{!}} Biology of Aging |url=https://courses.lumenlearning.com/atd-herkimer-biologyofaging/chapter/theory-3-cross-linkage-theory/ |website=courses.lumenlearning.com |access-date=7 October 2021}}</ref> || |
|- | |- | ||
| 1945 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The idea that {{w|free radical}}s are toxic agents is first proposed by Argentinian biologist {{w|Rebeca Gerschman}} and colleagues.<ref>{{cite journal |last1=Gerschman |first1=R. |last2=Gilbert |first2=D. L. |last3=Nye |first3=S. W. |last4=Dwyer |first4=P. |last5=Fenn |first5=W. O. |title=Oxygen Poisoning and X-irradiation: A Mechanism in Common |journal=Science |date=1954-05-07 |volume=119 |issue=3097 |pages=623–626 |doi=10.1126/science.119.3097.623}}</ref> || | | 1945 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The idea that {{w|free radical}}s are toxic agents is first proposed by Argentinian biologist {{w|Rebeca Gerschman}} and colleagues.<ref>{{cite journal |last1=Gerschman |first1=R. |last2=Gilbert |first2=D. L. |last3=Nye |first3=S. W. |last4=Dwyer |first4=P. |last5=Fenn |first5=W. O. |title=Oxygen Poisoning and X-irradiation: A Mechanism in Common |journal=Science |date=1954-05-07 |volume=119 |issue=3097 |pages=623–626 |doi=10.1126/science.119.3097.623}}</ref> || | ||
| Line 147: | Line 90: | ||
| 1945 || Organization || || The {{w|Gerontological Society of America}} is founded "to promote the scientific study of aging, to encourage exchanges among researchers and practitioners from the various disciplines related to gerontology, and to foster the use of gerontological research in forming public policy".<ref>{{cite web |title=The Gerontological Society of America |url=https://academic.oup.com/gsa/pages/about_gsa |website=Oxford Academic |access-date=2 July 2021 |language=en}}</ref> || {{w|United States}} | | 1945 || Organization || || The {{w|Gerontological Society of America}} is founded "to promote the scientific study of aging, to encourage exchanges among researchers and practitioners from the various disciplines related to gerontology, and to foster the use of gerontological research in forming public policy".<ref>{{cite web |title=The Gerontological Society of America |url=https://academic.oup.com/gsa/pages/about_gsa |website=Oxford Academic |access-date=2 July 2021 |language=en}}</ref> || {{w|United States}} | ||
|- | |- | ||
| − | | 1945–1949 || Scientific development || Laboratory || The advent of {{w|molecular biology}} changes the theoretical perception of aging dramatically, as the precise molecular structure of {{w|proteins}} and genetic material becomes known.<ref name=ilia-stambler/> || {{w|United States}} | + | | 1945–1949 || Scientific development || Laboratory research || The advent of {{w|molecular biology}} changes the theoretical perception of aging dramatically, as the precise molecular structure of {{w|proteins}} and genetic material becomes known.<ref name=ilia-stambler/> || {{w|United States}} |
|- | |- | ||
| 1946 || Literature (journal) || || The ''{{w|Journal of Gerontology}}'' is founded.<ref>{{cite web |title=Journal of Gerontology {{!}} Oxford Academic |url=https://academic.oup.com/geronj |website=OUP Academic |access-date=2 July 2021 |language=en}}</ref> || {{w|United States}} | | 1946 || Literature (journal) || || The ''{{w|Journal of Gerontology}}'' is founded.<ref>{{cite web |title=Journal of Gerontology {{!}} Oxford Academic |url=https://academic.oup.com/geronj |website=OUP Academic |access-date=2 July 2021 |language=en}}</ref> || {{w|United States}} | ||
|- | |- | ||
| − | | 1947 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Bogomolets links aging with the functional deterioration of connective tissues.<ref name="Medvedev"/> || | + | | 1947 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || {{w|Aleksandr Bogomolets}} links aging with the functional deterioration of connective tissues.<ref name="Medvedev"/> || |
|- | |- | ||
| 1950 || Organization || || The International Association of Gerontology is formed.<ref>{{cite web |title=About the IAG |url=https://www.sfu.ca/iag/about/index.htm |website=www.sfu.ca |access-date=12 July 2021}}</ref> || | | 1950 || Organization || || The International Association of Gerontology is formed.<ref>{{cite web |title=About the IAG |url=https://www.sfu.ca/iag/about/index.htm |website=www.sfu.ca |access-date=12 July 2021}}</ref> || | ||
|- | |- | ||
| − | | 1952 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || British biologist {{w|Peter Medawar}} formulates the first modern theory of mammal aging, known as {{w|Mutation accumulation theory}}, whereby the mechanism of action involves random, detrimental ''germline'' mutations of a kind that happen to show their effect only late in life.<ref name=the-evolution-of-aging/> || || [[File:Peter Brian Medawar.jpg|thumb|center|100px]] | + | | 1952 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || British biologist {{w|Peter Medawar}} formulates the first modern theory of mammal aging, known as {{w|Mutation accumulation theory}}, whereby the mechanism of action involves random, detrimental ''germline'' mutations of a kind that happen to show their effect only late in life.<ref name=the-evolution-of-aging/> || || [[File:Peter Brian Medawar.jpg|thumb|center|100px|{{w|Peter Medawar}}]] |
|- | |- | ||
| − | | 1954 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Soviet scientist {{w|Vladimir Dilman}} formulates the elevation hypothesis of aging, a hypothesis known only in the USSR at first. Later, in 1968, it would take the form and became known as the neuroendocrine theory of aging.<ref>{{cite journal|author=Ward Dean |url=https://warddeanmd.com/articles/neuroendocrine-theory-of-aging-chapter-1/ |title=Neuroendocrine Theory of Aging |website=warddeanmd.com |date=22 March 2012 |access-date=12 July 2021}}</ref><ref>{{cite journal |last1=Dilman |first1=V.M. |title=AGE-ASSOCIATED ELEVATION OF HYPOTHALAMIC THRESHOLD TO FEEDBACK CONTROL, AND ITS ROLE IN DEVELOPMENT, AGEING, AND DISEASE |journal=The Lancet |date=June 1971 |volume=297 |issue=7711 |pages=1211–1219 |doi=10.1016/s0140-6736(71)91721-1}}</ref><ref>{{cite journal |last1=Dilman |first1=V.M. |last2=Revskoy |first2=S.Y. |last3=Golubev |first3=A.G. |title=Neuroendocrine-Ontogenetic Mechanism of Aging: Toward An Integrated Theory of Aging |journal=International Review of Neurobiology |date=1986 |volume=28 |pages=89–156 |doi=10.1016/S0074-7742(08)60107-5}}</ref> || {{w|Russia}} ({{w|USSR}} | + | | 1954 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Soviet scientist {{w|Vladimir Dilman}} formulates the elevation hypothesis of aging, a hypothesis known only in the USSR at first. Later, in 1968, it would take the form and became known as the neuroendocrine theory of aging.<ref>{{cite journal|author=Ward Dean |url=https://warddeanmd.com/articles/neuroendocrine-theory-of-aging-chapter-1/ |title=Neuroendocrine Theory of Aging |website=warddeanmd.com |date=22 March 2012 |access-date=12 July 2021}}</ref><ref>{{cite journal |last1=Dilman |first1=V.M. |title=AGE-ASSOCIATED ELEVATION OF HYPOTHALAMIC THRESHOLD TO FEEDBACK CONTROL, AND ITS ROLE IN DEVELOPMENT, AGEING, AND DISEASE |journal=The Lancet |date=June 1971 |volume=297 |issue=7711 |pages=1211–1219 |doi=10.1016/s0140-6736(71)91721-1}}</ref><ref>{{cite journal |last1=Dilman |first1=V.M. |last2=Revskoy |first2=S.Y. |last3=Golubev |first3=A.G. |title=Neuroendocrine-Ontogenetic Mechanism of Aging: Toward An Integrated Theory of Aging |journal=International Review of Neurobiology |date=1986 |volume=28 |pages=89–156 |doi=10.1016/S0074-7742(08)60107-5}}</ref> || {{w|Russia}} ({{w|USSR}}) |
|- | |- | ||
| 1954 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The ''{{w|Free-radical theory of aging}}'' (first introduced by Dr. {{w|Rebeca Gerschman}}) is developed by Dr. Denham Harman, this theory proposes that superoxide and other free radicals cause damage to the macromolecular components of the cell, giving rise to accumulated damage causing cells, and eventually organs, to stop functioning.<ref name="kunlin">{{cite journal |last1=Jin |first1=Kunlin |title=Modern Biological Theories of Aging |journal=Aging and Disease |date=2010-08-01 |volume=1 |issue=2 |pages=72–74 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995895/ |issn=2152-5250}}</ref> || | | 1954 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The ''{{w|Free-radical theory of aging}}'' (first introduced by Dr. {{w|Rebeca Gerschman}}) is developed by Dr. Denham Harman, this theory proposes that superoxide and other free radicals cause damage to the macromolecular components of the cell, giving rise to accumulated damage causing cells, and eventually organs, to stop functioning.<ref name="kunlin">{{cite journal |last1=Jin |first1=Kunlin |title=Modern Biological Theories of Aging |journal=Aging and Disease |date=2010-08-01 |volume=1 |issue=2 |pages=72–74 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995895/ |issn=2152-5250}}</ref> || | ||
| Line 167: | Line 110: | ||
| 1956 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Benefitted by the cosolidation of knowledge of the toxic intermediate products of normal metabolism, {{w|Vladimir Korenchevsky}} introduces a general toxic theory of aging.<ref name="Medvedev"/> || | | 1956 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Benefitted by the cosolidation of knowledge of the toxic intermediate products of normal metabolism, {{w|Vladimir Korenchevsky}} introduces a general toxic theory of aging.<ref name="Medvedev"/> || | ||
|- | |- | ||
| − | | 1957 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || American evolutionary biologist [[wikipedia:George C. Williams (biologist)|George C. Williams]] proposes the today called {{w|Antagonistic pleiotropy hypothesis}} (AP) for the evolution of aging. It occurs when one gene controls for more than one phenotypic trait where at least one of these is beneficial to the organism's fitness and at least one is detrimental, thus accumulating damage.<ref name=the-evolution-of-aging/><ref name="williams">{{cite journal|doi=10.2307/2406060 |author=Williams, G.C.|title=Pleiotropy, natural selection and the evolution of senescence|journal=Evolution|volume=11|issue=4|pages=398–411 |year=1957|format=PDF|url=http://www.telomere.org/Downloads/Williams_searchable.pdf|jstor=2406060}} Paper in which Williams describes his theory of antagonistic pleiotropy.</ref> || {{w|United States}} | + | | 1956 || Scientific development || Laboratory research || Puck and Markus solve the problem of identifying all forms of cell death, by developing an assay based on the ability of a single cell to grow into a colony. This "clonogenic assay" would form the basis of in vitro cellular response studies in tumors and also some normal tissues.<ref name="Gewirtz"/> || |
| + | |- | ||
| + | | 1957 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || American evolutionary biologist [[wikipedia:George C. Williams (biologist)|George C. Williams]] proposes the today called {{w|Antagonistic pleiotropy hypothesis}} (AP) for the evolution of aging. It occurs when one gene controls for more than one phenotypic trait where at least one of these is beneficial to the organism's fitness and at least one is detrimental, thus accumulating damage.<ref name=the-evolution-of-aging/><ref name="williams">{{cite journal|doi=10.2307/2406060 |author=Williams, G.C.|title=Pleiotropy, natural selection and the evolution of senescence|journal=Evolution|volume=11|issue=4|pages=398–411 |year=1957|format=PDF|url=http://www.telomere.org/Downloads/Williams_searchable.pdf|jstor=2406060}} Paper in which Williams describes his theory of antagonistic pleiotropy.</ref> This theory extends the argument by {{w|Peter Medawar}}.<ref name="Shefferson"/> || {{w|United States}} | ||
|- | |- | ||
| 1957 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || M. F. Sinex publishes article titled ''Aging and the lability of irreplacable molecules'', establishing a theory based on quantitative changes of proteins, and describing as the loss of irreplaceable molecules or enzymes.<ref name="Medvedev"/><ref>{{cite web |last1=LAMBREMQNT |first1=EDWARD NELSON |title=A STUDY OF THE BIOCHEMISTRY AND PHYSIOLOGICAL AGING OF TWO PHOSPHATASES OF THE MOSQUITO, AEDES AEGYPTI |url=https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=osu1486575236787034&disposition=inline}}</ref> || | | 1957 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || M. F. Sinex publishes article titled ''Aging and the lability of irreplacable molecules'', establishing a theory based on quantitative changes of proteins, and describing as the loss of irreplaceable molecules or enzymes.<ref name="Medvedev"/><ref>{{cite web |last1=LAMBREMQNT |first1=EDWARD NELSON |title=A STUDY OF THE BIOCHEMISTRY AND PHYSIOLOGICAL AGING OF TWO PHOSPHATASES OF THE MOSQUITO, AEDES AEGYPTI |url=https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=osu1486575236787034&disposition=inline}}</ref> || | ||
| Line 187: | Line 132: | ||
| 1963 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Leslie Orgel proposes the Error Catastrophe Theory of Aging, which is based on changes in {{w|protein biosynthesis}} (translation).<ref name="Medvedev"/> This theory states that aging is the result of the accumulation of errors in cellular molecules that are essential for cellular function and reproduction that eventually reaches a catastrophic level that is incompatible with cellular survival.<ref>{{cite journal |last1=Diggs |first1=Jessica |title=The Error Catastrophe (Accumulation) Theory of Aging |journal=Encyclopedia of Aging and Public Health |date=2008 |pages=329–330 |doi=10.1007/978-0-387-33754-8_162}}</ref><ref name="Grossman">{{cite web |last1=Lange |first1=Jean |last2=Grossman |first2=Sheila |title=Theories of aging |url=https://samples.jbpub.com/9781284104479/Chapter_3.pdf |website=samples.jbpub.com}}</ref> || | | 1963 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Leslie Orgel proposes the Error Catastrophe Theory of Aging, which is based on changes in {{w|protein biosynthesis}} (translation).<ref name="Medvedev"/> This theory states that aging is the result of the accumulation of errors in cellular molecules that are essential for cellular function and reproduction that eventually reaches a catastrophic level that is incompatible with cellular survival.<ref>{{cite journal |last1=Diggs |first1=Jessica |title=The Error Catastrophe (Accumulation) Theory of Aging |journal=Encyclopedia of Aging and Public Health |date=2008 |pages=329–330 |doi=10.1007/978-0-387-33754-8_162}}</ref><ref name="Grossman">{{cite web |last1=Lange |first1=Jean |last2=Grossman |first2=Sheila |title=Theories of aging |url=https://samples.jbpub.com/9781284104479/Chapter_3.pdf |website=samples.jbpub.com}}</ref> || | ||
|- | |- | ||
| − | | 1963 || Scientific development || Laboratory || South African biologist {{w|Sydney Brenner}} suggests the ability to easily and cheaply grow large quantities of worms in the lab as being very helpful for aging research, especially when identifying long-lived mutants ''{{w|caenorhabditis elegans}}'', which have a relatively short lifespan (average approximately 17 days at 20 °C), and the lifespan is largely invariant.<ref name="Using C. elega"/> || || [[File:Sydney Brenner OIST 2008 (33208371153) (cropped) (2).jpg|thumb|center|100px|{{w|Sydney Brenner}}]] | + | | 1963 || Scientific development || Laboratory research || South African biologist {{w|Sydney Brenner}} suggests the ability to easily and cheaply grow large quantities of worms in the lab as being very helpful for aging research, especially when identifying long-lived mutants ''{{w|caenorhabditis elegans}}'', which have a relatively short lifespan (average approximately 17 days at 20 °C), and the lifespan is largely invariant.<ref name="Using C. elega"/> || || [[File:Sydney Brenner OIST 2008 (33208371153) (cropped) (2).jpg|thumb|center|100px|{{w|Sydney Brenner}}]] |
|- | |- | ||
| − | | 1964 || Scientific development || Concept introduction || The concept of {{w|programmed cell | + | | 1964 || Scientific development || Concept introduction || The concept of {{w|programmed cell death}} is introduced by American cellular biologist {{w|Richard A. Lockshin}} and Carroll Williams.<ref name="insect"> |
{{cite journal | {{cite journal | ||
|vauthors=Lockshin RA, Williams CM |year=1964 | |vauthors=Lockshin RA, Williams CM |year=1964 | ||
| Line 196: | Line 141: | ||
|volume=10 |issue=4 |pages=643–649 | |volume=10 |issue=4 |pages=643–649 | ||
|doi=10.1016/0022-1910(64)90034-4 | |doi=10.1016/0022-1910(64)90034-4 | ||
| − | }}</ref> || {{w|United States}} || [[File:Richard A. Lockshin.jpg|thumb|center|110px|Richard A. Lockshin]] | + | }}</ref> PCD consists in any active cellular process that culminates in cell death.<ref>{{cite web |title=Programmed Cell Death - an overview {{!}} ScienceDirect Topics |url=https://www.sciencedirect.com/topics/medicine-and-dentistry/programmed-cell-death |website=www.sciencedirect.com |access-date=7 October 2021}}</ref> || {{w|United States}} || [[File:Richard A. Lockshin.jpg|thumb|center|110px|Richard A. Lockshin]] |
|- | |- | ||
| 1964 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Romanian biochemist Simion Oeriu introduces a theory of aging based on age changes, described as accumulation of -S-S, inter- and intra- molecular bonds.<ref name="Medvedev"/> || {{w|Romania}} | | 1964 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Romanian biochemist Simion Oeriu introduces a theory of aging based on age changes, described as accumulation of -S-S, inter- and intra- molecular bonds.<ref name="Medvedev"/> || {{w|Romania}} | ||
|- | |- | ||
| + | | 1964 || Literature || || British scientist {{w|Alex Comfort}} publishes ''Ageing – the Biology of Senescence'', a classic book in the field. The book summarizes the state of the evolutionary study of senescence as lacking the scientific rigor seen in other disciplines. Comfort criticizes the field for yielding a number of evolutionary theories but failing to generate sufficient data to test those theories.<ref name="Shefferson"/> || | ||
| + | |- | ||
| 1965 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || {{w|Leonard Hayflick}} describes cell senescence as the process that limits the number of cell divisions normal human cells can undergo in culture. This work formulates the The Cellular Senescence Theory of aging.<ref name="ddsdd">{{cite journal |last1=Weinert |first1=Brian T. |last2=Timiras |first2=Poala S. |title=Invited Review: Theories of aging |journal=Journal of Applied Physiology |date=October 2003 |volume=95 |issue=4 |pages=1706–1716 |doi=10.1152/japplphysiol.00288.2003}}</ref><ref>{{cite journal |last1=Hayflick |first1=L. |title=The limited in vitro lifetime of human diploid cell strains |journal=Experimental Cell Research |date=March 1965 |volume=37 |issue=3 |pages=614–636 |doi=10.1016/0014-4827(65)90211-9}}</ref> || | | 1965 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || {{w|Leonard Hayflick}} describes cell senescence as the process that limits the number of cell divisions normal human cells can undergo in culture. This work formulates the The Cellular Senescence Theory of aging.<ref name="ddsdd">{{cite journal |last1=Weinert |first1=Brian T. |last2=Timiras |first2=Poala S. |title=Invited Review: Theories of aging |journal=Journal of Applied Physiology |date=October 2003 |volume=95 |issue=4 |pages=1706–1716 |doi=10.1152/japplphysiol.00288.2003}}</ref><ref>{{cite journal |last1=Hayflick |first1=L. |title=The limited in vitro lifetime of human diploid cell strains |journal=Experimental Cell Research |date=March 1965 |volume=37 |issue=3 |pages=614–636 |doi=10.1016/0014-4827(65)90211-9}}</ref> || | ||
|- | |- | ||
| − | | 1965–1969 || Scientific development || Laboratory || The strong effect of age on {{w|DNA methylation}} levels is discovered,<ref>{{cite journal | last1 = Berdyshev | first1 = G | last2 = Korotaev | first2 = G | last3 = Boiarskikh | first3 = G | last4 = Vaniushin | first4 = B | year = 1967 | title = Nucleotide composition of DNA and RNA from somatic tissues of humpback and its changes during spawning | url = | journal = Biokhimiia | volume = 31 | issue = | pages = 88–993 }}</ref> thus rendering it an accurate biological clock in humans and chimpanzees.<ref>{{Cite journal |author=Horvath S |title=DNA methylation age of human tissues and cell types |journal=Genome Biology |volume=14 |number=R115 |pages= R115|year=2013 |url=http://genomebiology.com/2013/14/10/r115|doi=10.1186/gb-2013-14-10-r115|pmid=24138928 |pmc=4015143}}</ref> || | + | | 1965–1969 || Scientific development || Laboratory research || The strong effect of age on {{w|DNA methylation}} levels is discovered,<ref>{{cite journal | last1 = Berdyshev | first1 = G | last2 = Korotaev | first2 = G | last3 = Boiarskikh | first3 = G | last4 = Vaniushin | first4 = B | year = 1967 | title = Nucleotide composition of DNA and RNA from somatic tissues of humpback and its changes during spawning | url = | journal = Biokhimiia | volume = 31 | issue = | pages = 88–993 }}</ref> thus rendering it an accurate biological clock in humans and chimpanzees.<ref>{{Cite journal |author=Horvath S |title=DNA methylation age of human tissues and cell types |journal=Genome Biology |volume=14 |number=R115 |pages= R115|year=2013 |url=http://genomebiology.com/2013/14/10/r115|doi=10.1186/gb-2013-14-10-r115|pmid=24138928 |pmc=4015143}}</ref> || |
|- | |- | ||
| − | | 1966 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || | + | | 1966 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || William Hamilton lays out the mathematics drawing on the ideas of [[wikipedia:George C. Williams (biologist)|George C. Williams]] and {{w|Peter Medawar}}, leading to the outcome that "senescence is an inevitable outcome of evolution".<ref name="Shefferson"/> || |
|- | |- | ||
| 1967 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || C. Alexander sets the grounds of the {{w|DNA damage theory of aging}} by suggesting that DNA damage, as distinct from mutation, is the primary cause of aging.<ref>{{cite journal |author=Alexander P |title=The role of DNA lesions in the processes leading to aging in mice |journal=Symp. Soc. Exp. Biol. |volume=21 |pages=29–50 |year=1967 |pmid=4860956 }}</ref> This theory becomes stronger through further experimental support during the following decades.<ref>{{cite book |vauthors=Bernstein C, Bernstein H |title=Aging, Sex, and DNA Repair |publisher=Academic Press |location=San Diego CA |year=1991 |isbn=0123960037 }}</ref><ref>{{cite journal |vauthors=Ames BN, Gold LS |title=Endogenous mutagens and the causes of aging and cancer |journal=Mutat. Res. |volume=250 |issue=1-2 |pages=3–16 |year=1991 |pmid=1944345 |doi=10.1016/0027-5107(91)90157-j}}</ref> || | | 1967 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || C. Alexander sets the grounds of the {{w|DNA damage theory of aging}} by suggesting that DNA damage, as distinct from mutation, is the primary cause of aging.<ref>{{cite journal |author=Alexander P |title=The role of DNA lesions in the processes leading to aging in mice |journal=Symp. Soc. Exp. Biol. |volume=21 |pages=29–50 |year=1967 |pmid=4860956 }}</ref> This theory becomes stronger through further experimental support during the following decades.<ref>{{cite book |vauthors=Bernstein C, Bernstein H |title=Aging, Sex, and DNA Repair |publisher=Academic Press |location=San Diego CA |year=1991 |isbn=0123960037 }}</ref><ref>{{cite journal |vauthors=Ames BN, Gold LS |title=Endogenous mutagens and the causes of aging and cancer |journal=Mutat. Res. |volume=250 |issue=1-2 |pages=3–16 |year=1991 |pmid=1944345 |doi=10.1016/0027-5107(91)90157-j}}</ref> || | ||
|- | |- | ||
| − | | 1969 || | + | | 1969 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || American physician {{w|Roy Walford}} introduces the autoimmune theory of aging, which asserts that with age, the immune system tends to lose efficiency and experiences widespread dysfunction, evidenced by autoimmunity (immune reactions against one's own body proteins) and a decreased ability to respond to infection and other immune challenges.<ref>{{cite journal |last1=Diggs |first1=Jessica |title=Autoimmune Theory of Aging |journal=Encyclopedia of Aging and Public Health |date=2008 |pages=143–144 |doi=10.1007/978-0-387-33754-8_46}}</ref> It is well documented that the effectiveness of the immune system peaks at puberty and gradually declines thereafter with advance in age.<ref>{{cite web |title=Theory 3: Autoimmune Theory {{!}} Biology of Aging |url=https://courses.lumenlearning.com/atd-herkimer-biologyofaging/chapter/theory-8-autoimmune-theory/ |website=courses.lumenlearning.com |access-date=7 October 2021}}</ref> || {{w|United States}} |
|- | |- | ||
| − | | 1970 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Selye introduces the Stress damage theory, | + | | 1970 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Hungarian-Canadian endocrinologist {{w|Hans Selye}} introduces the Stress damage theory, after observing a series of physiological changes in the rats after they were exposed to stressful events. Considered an important theory of aging at the whole animal level, this theory identifies three stages: alarm, resistance, and exhaustion.<ref name="Dazhong"/><ref>{{cite web |title=General Adaptation Syndrome: Your Body’s Response to Stress |url=https://www.healthline.com/health/general-adaptation-syndrome |website=Healthline |access-date=23 December 2021 |language=en |date=1 May 2017}}</ref> || {{w|Canada}} || [[File:Portrait Hans Selye.jpg|thumb|center|100px|{{w|Hans Selye}}]] |
|- | |- | ||
| 1970 || Organization || || The {{w|American Aging Association}} is founded by {{w|Denham Harman}}. It is a {{w|non-profit}}, tax-exempt {{w|biogerontology}} {{w|organization}} of scientists and [[w:Layperson|laypeople]] dedicated to [[w:Health science|biomedical]] [[w:ageing|aging]] studies and {{w|geroscience}}, with the goal of slowing the [[w:Senescence|aging process]] to extend the healthy human lifespan while preserving and restoring functions typically lost to age-related degeneration.<ref>{{cite web |last1=Ocaklı |first1=Burcu Özdemir |title=American Aging Association |url=https://link.springer.com/referenceworkentry/10.1007%2F978-3-319-69892-2_218-1 |website=Encyclopedia of Gerontology and Population Aging |publisher=Springer International Publishing |access-date=15 June 2021 |pages=1–5 |language=en |doi=10.1007/978-3-319-69892-2_218-1 |date=2019}}</ref> || {{w|United States}} | | 1970 || Organization || || The {{w|American Aging Association}} is founded by {{w|Denham Harman}}. It is a {{w|non-profit}}, tax-exempt {{w|biogerontology}} {{w|organization}} of scientists and [[w:Layperson|laypeople]] dedicated to [[w:Health science|biomedical]] [[w:ageing|aging]] studies and {{w|geroscience}}, with the goal of slowing the [[w:Senescence|aging process]] to extend the healthy human lifespan while preserving and restoring functions typically lost to age-related degeneration.<ref>{{cite web |last1=Ocaklı |first1=Burcu Özdemir |title=American Aging Association |url=https://link.springer.com/referenceworkentry/10.1007%2F978-3-319-69892-2_218-1 |website=Encyclopedia of Gerontology and Population Aging |publisher=Springer International Publishing |access-date=15 June 2021 |pages=1–5 |language=en |doi=10.1007/978-3-319-69892-2_218-1 |date=2019}}</ref> || {{w|United States}} | ||
| + | |- | ||
| + | | 1972 || Scientific development || Concept development || Kerr, Wyllie, and Currie first coin the term “apoptosis” to differentiate naturally occurring developmental cell death from the {{w|necrosis}} that results from acute tissue injury. They also note that apoptosis is responsible for maintaining tissue {{w|homeostasis}} by mediating the equilibrium between cell proliferation and cell death in a particular tissue.<ref name="Gewirtz">{{cite book |last1=Gewirtz |first1=David A. |last2=Holt |first2=Shawn E. |last3=Grant |first3=Steven |title=Apoptosis, Senescence and Cancer |date=23 October 2007 |publisher=Springer Science & Business Media |isbn=978-1-59745-221-2 |url=https://books.google.com.ar/books/about/Apoptosis_Senescence_and_Cancer.html?id=2vrQfUc4oz4C&source=kp_book_description&redir_esc=y |language=en}}</ref> || | ||
|- | |- | ||
| 1974 || Organization || || The {{w|National Institute on Aging}} (NIA) is formed as a division of the United States [[wikipedia:National Institutes of Health|National Institutes of Health]] (NIH), with the purpose of conducting research on aging process and age-related diseases and disseminating information on health and research advances, among other aims.<ref>{{cite book|last1=Ofahengaue Vakalahi|first1=Halaevalu F.|last2=Simpson|first2=Gaynell M.|last3=Giunta|first3=Nancy|title=The Collective Spirit of Aging Across Cultures|page=20|url=https://books.google.com.ar/books?id=58XEBAAAQBAJ&pg=PA20&lpg=PA20&dq=%221974%22+%22National+Institute+on+Aging+%22&source=bl&ots=h5rgitHiKf&sig=W29lqL0xqia6j_EdPOGrioIvd58&hl=en&sa=X&ved=0ahUKEwi6kuXq9oXRAhXLDJAKHYgwA-oQ6AEINzAF#v=onepage&q=%221974%22%20%22National%20Institute%20on%20Aging%20%22&f=false|accessdate=21 December 2016}}</ref><ref>{{cite web |url=https://www.nia.nih.gov/ |title=National Institute of Aging}}</ref> || {{w|United States}} ({{w|National Institute on Aging}}) | | 1974 || Organization || || The {{w|National Institute on Aging}} (NIA) is formed as a division of the United States [[wikipedia:National Institutes of Health|National Institutes of Health]] (NIH), with the purpose of conducting research on aging process and age-related diseases and disseminating information on health and research advances, among other aims.<ref>{{cite book|last1=Ofahengaue Vakalahi|first1=Halaevalu F.|last2=Simpson|first2=Gaynell M.|last3=Giunta|first3=Nancy|title=The Collective Spirit of Aging Across Cultures|page=20|url=https://books.google.com.ar/books?id=58XEBAAAQBAJ&pg=PA20&lpg=PA20&dq=%221974%22+%22National+Institute+on+Aging+%22&source=bl&ots=h5rgitHiKf&sig=W29lqL0xqia6j_EdPOGrioIvd58&hl=en&sa=X&ved=0ahUKEwi6kuXq9oXRAhXLDJAKHYgwA-oQ6AEINzAF#v=onepage&q=%221974%22%20%22National%20Institute%20on%20Aging%20%22&f=false|accessdate=21 December 2016}}</ref><ref>{{cite web |url=https://www.nia.nih.gov/ |title=National Institute of Aging}}</ref> || {{w|United States}} ({{w|National Institute on Aging}}) | ||
| Line 220: | Line 169: | ||
| 1975 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Sacher introduces a brain size–lifespan correlation theory, suggesting that larger brains make evolutionary selection of longer lifespan necessary.<ref name="Medvedev"/> || | | 1975 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Sacher introduces a brain size–lifespan correlation theory, suggesting that larger brains make evolutionary selection of longer lifespan necessary.<ref name="Medvedev"/> || | ||
|- | |- | ||
| − | | 1975 || Scientific development || Laboratory || Australian-American molecular biologist {{w|Elizabeth Blackburn}} discovers the unusual nature of {{w|telomere}}s, with their simple repeated DNA sequences composing chromosome ends.<ref>{{cite web|url=http://www.ibiology.org/ibioseminars/genetics-gene-regulation/elizabeth-blackburn-part-1.html|title=ELIZABETH BLACKBURN: TELOMERES AND TELOMERASE}}</ref><ref>{{cite journal |author=Blackburn AM |title=A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena |journal=J. Mol. Biol. |volume=120 |issue=1 |pages=33–53 | date=March 1978 |doi=10.1016/0022-2836(78)90294-2 |pmid=642006 |first2=Joseph G.|last2=Gall }}</ref> Some years later, Blackburn, [[wikipedia:Carol Greider|Carol Greider]] and [[wikipedia:Jack Szostak|Jack Szostak]] discover how chromosomes are protected by telomeres and the enzyme [[wikipedia:telomerase|telomerase]], for which they receive the 2009 Nobel Prize in Physiology or Medicine.<ref>{{cite web|url=http://nobelprize.org/nobel_prizes/medicine/laureates/2009/press.html |title=The 2009 Nobel Prize in Physiology or Medicine - Press Release |publisher=Nobelprize.org |date=2009-10-05 |accessdate=2012-06-12}}</ref> Further experiments establish the role of telomere shortening in [[wikipedia:cellular aging|cellular aging]] and telomerase reactivation in cell immortalization.<ref>{{cite news|title=Unravelling the secret of aging |url=http://www.cosmosmagazine.com/features/unraveling-secret-aging/ |work=COSMOS: The Science of Everything |date=October 5, 2009 |deadurl=yes |archiveurl=https://web.archive.org/web/20150114212810/http://cosmosmagazine.com/features/unraveling-secret-aging/ |archivedate=January 14, 2015 }}</ref> || {{w|United States}} || [[File:Elizabeth Blackburn 2009-01.JPG|thumb|center|100px|{{w|Elizabeth Blackburn}}]] | + | | 1975 || Scientific development || Laboratory research || Australian-American molecular biologist {{w|Elizabeth Blackburn}} discovers the unusual nature of {{w|telomere}}s, with their simple repeated DNA sequences composing chromosome ends.<ref>{{cite web|url=http://www.ibiology.org/ibioseminars/genetics-gene-regulation/elizabeth-blackburn-part-1.html|title=ELIZABETH BLACKBURN: TELOMERES AND TELOMERASE}}</ref><ref>{{cite journal |author=Blackburn AM |title=A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena |journal=J. Mol. Biol. |volume=120 |issue=1 |pages=33–53 | date=March 1978 |doi=10.1016/0022-2836(78)90294-2 |pmid=642006 |first2=Joseph G.|last2=Gall }}</ref> Some years later, Blackburn, [[wikipedia:Carol Greider|Carol Greider]] and [[wikipedia:Jack Szostak|Jack Szostak]] discover how chromosomes are protected by telomeres and the enzyme [[wikipedia:telomerase|telomerase]], for which they receive the 2009 Nobel Prize in Physiology or Medicine.<ref>{{cite web|url=http://nobelprize.org/nobel_prizes/medicine/laureates/2009/press.html |title=The 2009 Nobel Prize in Physiology or Medicine - Press Release |publisher=Nobelprize.org |date=2009-10-05 |accessdate=2012-06-12}}</ref> Further experiments establish the role of telomere shortening in [[wikipedia:cellular aging|cellular aging]] and telomerase reactivation in cell immortalization.<ref>{{cite news|title=Unravelling the secret of aging |url=http://www.cosmosmagazine.com/features/unraveling-secret-aging/ |work=COSMOS: The Science of Everything |date=October 5, 2009 |deadurl=yes |archiveurl=https://web.archive.org/web/20150114212810/http://cosmosmagazine.com/features/unraveling-secret-aging/ |archivedate=January 14, 2015 }}</ref> || {{w|United States}} || [[File:Elizabeth Blackburn 2009-01.JPG|thumb|center|100px|{{w|Elizabeth Blackburn}}]] |
|- | |- | ||
| − | | 1976 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Cutler introduces a theory of aging based on age changes, and | + | | 1976 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Cutler introduces a theory of aging based on age changes, and describes it as the cross-links between DNA molecules.<ref name="Medvedev"/> || |
|- | |- | ||
| − | | 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || English biologist {{w|Thomas Kirkwood}} proposes the third mainstream theory of aging, the {{w|disposable soma}}, which states that organisms only have a limited amount of energy that has to be divided between reproductive activities and the maintenance of the non-reproductive aspects of the organism.<ref>{{cite book |last=Goldsmith |first=Theodore |date= |title=The Evolution of Aging: How New Theories Will Change the Future of Medicine |url=https://books.google.com.ar/books?id=bCwC22mim4gC&pg=PA48&lpg=PA48&dq=%22Thomas+Kirkwood%22+%22Disposable+soma%22+%221977%22&source=bl&ots=JCdSwbtSVo&sig=Ht8Ttjq7KF_zPMvlhtxPbY_ZxHc&hl=en&sa=X&ved=0ahUKEwi5uqfCmqjOAhXHvJAKHWIlBrwQ6AEIPTAG#v=onepage&q=%22Thomas%20Kirkwood%22%20%22Disposable%20soma%22%20%221977%22&f=false |location= |publisher= |page=48 |isbn=}}</ref> Considered to be the most influential line of reasoning in gerontology, this theory states that aging is caused by accumulation of random damage, which is counteracted by repair. Repair is costly and the organism allocates exactly the needed amount of energetic resources. DST would be applied to explain why women live longer than men. Women are less disposable than men, so they need a better repair and thus live longer.<ref>{{cite journal |last1=Blagosklonny |first1=Mikhail V. |title=Why the disposable soma theory cannot explain why women live longer and why we age |journal=Aging |date=29 December 2010 |volume=2 |issue=12 |pages=884–887 |doi=10.18632/aging.100253}}</ref> || | + | | 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || English biologist {{w|Thomas Kirkwood}} proposes the third mainstream theory of aging, the {{w|disposable soma}}, which states that organisms only have a limited amount of energy that has to be divided between reproductive activities and the maintenance of the non-reproductive aspects of the organism.<ref>{{cite book |last=Goldsmith |first=Theodore |date= |title=The Evolution of Aging: How New Theories Will Change the Future of Medicine |url=https://books.google.com.ar/books?id=bCwC22mim4gC&pg=PA48&lpg=PA48&dq=%22Thomas+Kirkwood%22+%22Disposable+soma%22+%221977%22&source=bl&ots=JCdSwbtSVo&sig=Ht8Ttjq7KF_zPMvlhtxPbY_ZxHc&hl=en&sa=X&ved=0ahUKEwi5uqfCmqjOAhXHvJAKHWIlBrwQ6AEIPTAG#v=onepage&q=%22Thomas%20Kirkwood%22%20%22Disposable%20soma%22%20%221977%22&f=false |location= |publisher= |page=48 |isbn=}}</ref> Considered to be the most influential line of reasoning in gerontology, this theory states that aging is caused by accumulation of random damage, which is counteracted by repair. Repair is costly and the organism allocates exactly the needed amount of energetic resources. DST would be applied to explain why women live longer than men. Women are less disposable than men, so they need a better repair and thus live longer.<ref>{{cite journal |last1=Blagosklonny |first1=Mikhail V. |title=Why the disposable soma theory cannot explain why women live longer and why we age |journal=Aging |date=29 December 2010 |volume=2 |issue=12 |pages=884–887 |doi=10.18632/aging.100253}}</ref> This theory would be further developed in substantial detail (Flatt et al. 2013; Kirkwood 1981, 1985, 2002, 2005; Kirkwood & Holliday 1979; Kirkwood & Rose 1991).<ref name="Shefferson"/> || |
|- | |- | ||
| 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Research started by Kirkwood establishes an aging theory on lifespan correlations with changes at the molecular level. This theory suggests that immortal germ cells may have more comprehensive repairs and may have higher accuracy of synthesis than somatic cells.<ref name="Medvedev"/> || | | 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Research started by Kirkwood establishes an aging theory on lifespan correlations with changes at the molecular level. This theory suggests that immortal germ cells may have more comprehensive repairs and may have higher accuracy of synthesis than somatic cells.<ref name="Medvedev"/> || | ||
| Line 230: | Line 179: | ||
| 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Holliday and colleagues introduce the commitment theory of cellular aging, which stipulates the existence of a biological clock (pace-maker).<ref name="Medvedev"/> || | | 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Holliday and colleagues introduce the commitment theory of cellular aging, which stipulates the existence of a biological clock (pace-maker).<ref name="Medvedev"/> || | ||
|- | |- | ||
| − | | 1977 || Scientific development || Laboratory || Klass publishes that nematode ''{{w|C. elegans}}'' is a good system for aging studies as he establishes a method to consistently measure lifespan, concluding that this could lead to future detailed analysis combining genetics and biochemistry. Klass also finds that altering either temperature or the amount of food results in a change in lifespan. In addition, only small effects on lifespan are observed based on parental age or parental lifespan.<ref name="Using C. elega"/> || || | + | | 1977 || Scientific development || Laboratory research || Klass publishes that nematode ''{{w|C. elegans}}'' is a good system for aging studies as he establishes a method to consistently measure lifespan, concluding that this could lead to future detailed analysis combining genetics and biochemistry. Klass also finds that altering either temperature or the amount of food results in a change in lifespan. In addition, only small effects on lifespan are observed based on parental age or parental lifespan.<ref name="Using C. elega"/> || || |
|- | |- | ||
| 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || British biologist Thomas Kirkwood first proposes the disposable soma theory of aging in a Nature review article. This theory is inspired by Leslie Orgel's Error Catastrophe Theory of Aging.<ref>{{cite journal |last1=Sotola |first1=Lukas |title=Disposable Soma Theory |journal=Encyclopedia of Evolutionary Psychological Science |date=2016 |pages=1–4 |doi=10.1007/978-3-319-16999-6_2367-1 |url=}}</ref> || | | 1977 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || British biologist Thomas Kirkwood first proposes the disposable soma theory of aging in a Nature review article. This theory is inspired by Leslie Orgel's Error Catastrophe Theory of Aging.<ref>{{cite journal |last1=Sotola |first1=Lukas |title=Disposable Soma Theory |journal=Encyclopedia of Evolutionary Psychological Science |date=2016 |pages=1–4 |doi=10.1007/978-3-319-16999-6_2367-1 |url=}}</ref> || | ||
| Line 240: | Line 189: | ||
| 1978 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Soviet scientist A. N. Lobachev proposes the [[w:mitochondrial theory of aging| mitochondrial non free radical theory of aging]], which suggests that the main reason of accumulation of damages in mt DNA is the fact that at certain moment of cell life, the development of mitochondria begin to conflict with the development of nucleus. This theory concludes that mitochondria appears to be the «biologic clock» of the cell and programm the duration of its life.<ref>{{cite web |title=БИОГЕНЕЗ МИТОХОНДРИЙ ПРИ ДИФФЕРЕНЦИАЦИИ И СТАРЕНИИ КЛЕТОК |url=http://aiexandr2010.narod.ru/Biogenesis.pdf |website=aiexandr2010.narod.ru |access-date=10 June 2021}}</ref><ref name="Mitochondrial Theory of Aging">{{cite web |title=Mitochondrial Theory of Aging — IVAO |url=http://www.ivao.com/en/anti-aging/teorii-stareniya/mitokhondrialnaya-teoriya-stareniya/mitokhondrialnaya-teoriya-stareniya/ |website=www.ivao.com |access-date=10 June 2021}}</ref> || {{w|Russia}} || | | 1978 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Soviet scientist A. N. Lobachev proposes the [[w:mitochondrial theory of aging| mitochondrial non free radical theory of aging]], which suggests that the main reason of accumulation of damages in mt DNA is the fact that at certain moment of cell life, the development of mitochondria begin to conflict with the development of nucleus. This theory concludes that mitochondria appears to be the «biologic clock» of the cell and programm the duration of its life.<ref>{{cite web |title=БИОГЕНЕЗ МИТОХОНДРИЙ ПРИ ДИФФЕРЕНЦИАЦИИ И СТАРЕНИИ КЛЕТОК |url=http://aiexandr2010.narod.ru/Biogenesis.pdf |website=aiexandr2010.narod.ru |access-date=10 June 2021}}</ref><ref name="Mitochondrial Theory of Aging">{{cite web |title=Mitochondrial Theory of Aging — IVAO |url=http://www.ivao.com/en/anti-aging/teorii-stareniya/mitokhondrialnaya-teoriya-stareniya/mitokhondrialnaya-teoriya-stareniya/ |website=www.ivao.com |access-date=10 June 2021}}</ref> || {{w|Russia}} || | ||
|- | |- | ||
| − | | 1978 || Scientific development || Laboratory || For research on aging, early studies in nematode ''{{w|C. elegans}}'' focus on the feasibility of measuring lifespan and the use of 5-Fluoro-2′-deoxyuridine (FUDR) to maintain synchronous cultures of aged animals.<ref name="Using C. elega">{{cite journal |last1=Tissenbaum |first1=Heidi A. |title=Using C. elegans for aging research |journal=Invertebrate Reproduction & Development |date=2015-01-30 |volume=59 |issue=sup1 |pages=59–63 |doi=10.1080/07924259.2014.940470 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4464094/ |issn=0792-4259}}</ref> || || | + | | 1978 || Scientific development || Laboratory research || For research on aging, early studies in nematode ''{{w|C. elegans}}'' focus on the feasibility of measuring lifespan and the use of 5-Fluoro-2′-deoxyuridine (FUDR) to maintain synchronous cultures of aged animals.<ref name="Using C. elega">{{cite journal |last1=Tissenbaum |first1=Heidi A. |title=Using C. elegans for aging research |journal=Invertebrate Reproduction & Development |date=2015-01-30 |volume=59 |issue=sup1 |pages=59–63 |doi=10.1080/07924259.2014.940470 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4464094/ |issn=0792-4259}}</ref> || || |
| + | |- | ||
| + | | 1979 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Vaupel et al. produce one of the earliest quantitative theories of lifelong heterogeneity. Under their model, the probability of an individual dying is described by a Gompertz equation, a sigmoid function which describes growth as being slowest at the start and end of a given time period.<ref name="Shefferson"/> || | ||
|- | |- | ||
| 1979 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Research by D. Gershon at {{w|Technion}} introduces a theory of aging based on age changes, and described as age-altered enzymes theory (age-related accumulation of conformational changes in protein leading to the inactivation of enzymes).<ref name="Medvedev"/> || {{w|Israel}} | | 1979 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Research by D. Gershon at {{w|Technion}} introduces a theory of aging based on age changes, and described as age-altered enzymes theory (age-related accumulation of conformational changes in protein leading to the inactivation of enzymes).<ref name="Medvedev"/> || {{w|Israel}} | ||
| Line 248: | Line 199: | ||
| 1980 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || M. S. Kanungo introduces a theory of aging based on age changes, describing it as the decrease of phosphorylation and acetylation of chromatin proteins as the cause of defects and decline in transcription.<ref name="Medvedev"/><ref>{{cite journal |last1=Das |first1=R |last2=Kanungo |first2=MS |title=In vitro phosphorylation of chromosomal proteins of the brain of rats of various ages & its modulation by epinephrine. |journal=Indian journal of biochemistry & biophysics |date=June 1980 |volume=17 |issue=3 |pages=217-21 |pmid=7450808}}</ref> || | | 1980 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || M. S. Kanungo introduces a theory of aging based on age changes, describing it as the decrease of phosphorylation and acetylation of chromatin proteins as the cause of defects and decline in transcription.<ref name="Medvedev"/><ref>{{cite journal |last1=Das |first1=R |last2=Kanungo |first2=MS |title=In vitro phosphorylation of chromosomal proteins of the brain of rats of various ages & its modulation by epinephrine. |journal=Indian journal of biochemistry & biophysics |date=June 1980 |volume=17 |issue=3 |pages=217-21 |pmid=7450808}}</ref> || | ||
|- | |- | ||
| − | | 1980 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Strehler introduces an aging theory classification that underlines the interrelation between different mechanisms of aging in an attempt to unite all these types into two "supergroups": genetically programmed aging, and aging secondary to genetic qualities (entropic increase). Strehler classification | + | | 1980 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Strehler introduces an aging theory classification that underlines the interrelation between different mechanisms of aging in an attempt to unite all these types into two "supergroups": genetically programmed aging, and aging secondary to genetic qualities (entropic increase). Strehler classification groups theories of aging into evolutionary (selection for limited lifespan), post-reproductive failure, failure of coordinating systems, pleiotropic side effects of advantageous qualities (clonal aging, autoimmunity), informational failure (changes in proteins and DNA), structural damage or loss and accumulation of dysfunctional materials (age-pigments, inactive proteins, lytic enzymes, etc.)<ref name="Medvedev"/> || |
| + | |- | ||
| + | | 1981 || Scientific development || || Lee Eberhardt stresses the need of including senescence when assessing population dynamics, as senescence is expected to have strong implications for conservation and management of mammalian populations. In particular, the occurrence of actuarial and reproductive senescence is expected to lead to a decrease in individual fitness and population growth rates.<ref name="Shefferson"/> || | ||
|- | |- | ||
| 1982 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Pashko and Schwartz propose an aging theory on lifespan correlations at the cellular level, suggesting a correlation between lifespan and species-specific activity of detoxification enzymes (longer-lived animals have higher efficiency of detoxification and are more resistant to environmental toxins).<ref name="Medvedev"/> || | | 1982 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Pashko and Schwartz propose an aging theory on lifespan correlations at the cellular level, suggesting a correlation between lifespan and species-specific activity of detoxification enzymes (longer-lived animals have higher efficiency of detoxification and are more resistant to environmental toxins).<ref name="Medvedev"/> || | ||
|- | |- | ||
| − | | 1986 || Organization ([[w:Nonprofit organization|non-profit]]) || || The {{w|Alliance for Aging Research}} is founded a non-profit organization, with the purpose to promote medical and behavioral research into the aging process.<ref>{{cite web |title=Alliance for Aging Research |url=https://www.idealist.org/en/nonprofit/f8b1e4955c4a4f72bf767556801e96ff-alliance-for-aging-research-washington |website=www.idealist.org |access-date=8 June 2021 |language=en}}</ref> || {{w|United States}} | + | | 1986 || Organization ([[w:Nonprofit organization|non-profit]]) || || The {{w|Alliance for Aging Research}} is founded as a non-profit organization, with the purpose to promote medical and behavioral research into the aging process.<ref>{{cite web |title=Alliance for Aging Research |url=https://www.idealist.org/en/nonprofit/f8b1e4955c4a4f72bf767556801e96ff-alliance-for-aging-research-washington |website=www.idealist.org |access-date=8 June 2021 |language=en}}</ref> || {{w|United States}} |
|- | |- | ||
| 1986 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The {{w|Reliability theory of aging and longevity}} is proposed by Leonid Gavrilov and Natalia Gavrilova.<ref>{{cite book| vauthors = Gavrilov LA, Gavrilova NS |year=1986 | veditors = Skulachev WP |title=Биология продолжительности жизни: Количественные аспекты |language=ru |trans-title=Biology of Life Span: Quantitative Aspects |url=https://books.google.com/books?id=zMY5AAAAIAAJ |edition=1st |location=Moscow |publisher=[[w:Nauka (publisher)|Nauka]] |pages=167}}</ref><ref>{{cite book| vauthors = Gavrilov LA, Gavrilova NS | year=1991 | veditors = Skulachev VP|title=Biology of Life Span: A Quantitative Approach |language=en |edition=1st |location=New York |publisher=Chur |pages=385 |isbn=978-3718649839}}</ref><ref>{{cite journal | vauthors = Gavrilov LA, Gavrilova NS | title = The reliability theory of aging and longevity | journal = Journal of Theoretical Biology | volume = 213 | issue = 4 | pages = 527–45 | date = December 2001 | pmid = 11742523 | doi = 10.1006/jtbi.2001.2430 }}</ref><ref>{{cite journal|author=A.J.S. Rayl |date=13 May 2002 |title=Aging, in Theory: A Personal Pursuit. Do body system redundancies hold the key? |url=http://longevity-science.org/TheScientist.pdf |journal=[[w:The Scientist (magazine)|The Scientist]] |volume=16 |issue=10 |pages=20 |doi= |pmc= |pmid=}}</ref> || | | 1986 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The {{w|Reliability theory of aging and longevity}} is proposed by Leonid Gavrilov and Natalia Gavrilova.<ref>{{cite book| vauthors = Gavrilov LA, Gavrilova NS |year=1986 | veditors = Skulachev WP |title=Биология продолжительности жизни: Количественные аспекты |language=ru |trans-title=Biology of Life Span: Quantitative Aspects |url=https://books.google.com/books?id=zMY5AAAAIAAJ |edition=1st |location=Moscow |publisher=[[w:Nauka (publisher)|Nauka]] |pages=167}}</ref><ref>{{cite book| vauthors = Gavrilov LA, Gavrilova NS | year=1991 | veditors = Skulachev VP|title=Biology of Life Span: A Quantitative Approach |language=en |edition=1st |location=New York |publisher=Chur |pages=385 |isbn=978-3718649839}}</ref><ref>{{cite journal | vauthors = Gavrilov LA, Gavrilova NS | title = The reliability theory of aging and longevity | journal = Journal of Theoretical Biology | volume = 213 | issue = 4 | pages = 527–45 | date = December 2001 | pmid = 11742523 | doi = 10.1006/jtbi.2001.2430 }}</ref><ref>{{cite journal|author=A.J.S. Rayl |date=13 May 2002 |title=Aging, in Theory: A Personal Pursuit. Do body system redundancies hold the key? |url=http://longevity-science.org/TheScientist.pdf |journal=[[w:The Scientist (magazine)|The Scientist]] |volume=16 |issue=10 |pages=20 |doi= |pmc= |pmid=}}</ref> || | ||
|- | |- | ||
| − | | 1987 || Scientific development || Laboratory || B.M. Stanulis-Praeger determines cell death to be a primary consequence of senescence.<ref>{{cite journal |last1=Stanulis-Praeger |first1=B. M. |title=Cellular senescence revisited: a review |journal=Mechanisms of Aging and Development |date=March 1987 |volume=38 |issue=1 |pages=1–48 |doi=10.1016/0047-6374(87)90109-6 |url=https://pubmed.ncbi.nlm.nih.gov/2439851/ |issn=0047-6374}}</ref> || {{w|United States}} ({{w|Tufts University}}, Boston) || | + | | 1987 || Scientific development || Laboratory research || B.M. Stanulis-Praeger determines cell death to be a primary consequence of senescence.<ref>{{cite journal |last1=Stanulis-Praeger |first1=B. M. |title=Cellular senescence revisited: a review |journal=Mechanisms of Aging and Development |date=March 1987 |volume=38 |issue=1 |pages=1–48 |doi=10.1016/0047-6374(87)90109-6 |url=https://pubmed.ncbi.nlm.nih.gov/2439851/ |issn=0047-6374}}</ref> || {{w|United States}} ({{w|Tufts University}}, Boston) || |
|- | |- | ||
| − | | 1988 || Scientific development || Laboratory || Genetic work by Tom Johnson et al. on mutant {{w|C. elegans}} mapps all of them to a single genetic locus, named age-1. This is the first breakthrough in aging research for studies based on C. elegans as this study reveals that it is possible to identify mutants that altered lifespan and more importantly, individual genes can modulate lifespan.<ref name="Using C. elega"/> || | + | | 1988 || Scientific development || Laboratory research || Genetic work by Tom Johnson et al. on mutant {{w|C. elegans}} mapps all of them to a single genetic locus, named age-1. This is the first breakthrough in aging research for studies based on C. elegans as this study reveals that it is possible to identify mutants that altered lifespan and more importantly, individual genes can modulate lifespan.<ref name="Using C. elega"/> || |
|- | |- | ||
| − | | 1989 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The {{w|network theory of aging}} is introduced. It supports the idea that multiple connected processes contribute to the biology of aging.<ref name="Yashin">{{cite journal |last1=Franceschi |first1=C |last2=Valensin |first2=S |last3=Bonafè |first3=M |last4=Paolisso |first4=G |last5=Yashin |first5=A.I |last6=Monti |first6=D |last7=De Benedictis |first7=G |title=The network and the remodeling theories of aging: historical background and new perspectives |journal=Experimental Gerontology |date=September 2000 |volume=35 |issue=6-7 |pages=879–896 |doi=10.1016/S0531-5565(00)00172-8}}</ref> | + | | 1988 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || In a pioneering attempt to find quantitative evidence for senescence in wild populations of animals, Nesse concludes that it does in fact occur.<ref name="Shefferson"/> || |
| + | |- | ||
| + | | 1989 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The {{w|network theory of aging}} is introduced. It supports the idea that multiple connected processes contribute to the biology of aging.<ref name="Yashin">{{cite journal |last1=Franceschi |first1=C |last2=Valensin |first2=S |last3=Bonafè |first3=M |last4=Paolisso |first4=G |last5=Yashin |first5=A.I |last6=Monti |first6=D |last7=De Benedictis |first7=G |title=The network and the remodeling theories of aging: historical background and new perspectives |journal=Experimental Gerontology |date=September 2000 |volume=35 |issue=6-7 |pages=879–896 |doi=10.1016/S0531-5565(00)00172-8}}</ref> || | ||
|- | |- | ||
| 1990 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Russian biologist {{w|Zhores Medvedev}} publishes review stating that there are more than 300 theories of aging and the number is increasing.<ref name="Medvedev">{{cite journal |last1=Medvedev |first1=Zhores A. |title=AN ATTEMPT AT A RATIONAL CLASSIFICATION OF THEORIES OF AGING |journal=Biological Reviews |date=August 1990 |volume=65 |issue=3 |pages=375–398 |doi=10.1111/j.1469-185x.1990.tb01428.x}}</ref><ref>{{cite journal |last1=Viña |first1=Jose |last2=Borrás |first2=Consuelo |last3=Miquel |first3=Jaime |title=Theories of ageing |journal=IUBMB Life |date=2007 |volume=59 |issue=4 |pages=249–254 |doi=10.1080/15216540601178067}}</ref> || | | 1990 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Russian biologist {{w|Zhores Medvedev}} publishes review stating that there are more than 300 theories of aging and the number is increasing.<ref name="Medvedev">{{cite journal |last1=Medvedev |first1=Zhores A. |title=AN ATTEMPT AT A RATIONAL CLASSIFICATION OF THEORIES OF AGING |journal=Biological Reviews |date=August 1990 |volume=65 |issue=3 |pages=375–398 |doi=10.1111/j.1469-185x.1990.tb01428.x}}</ref><ref>{{cite journal |last1=Viña |first1=Jose |last2=Borrás |first2=Consuelo |last3=Miquel |first3=Jaime |title=Theories of ageing |journal=IUBMB Life |date=2007 |volume=59 |issue=4 |pages=249–254 |doi=10.1080/15216540601178067}}</ref> || | ||
| Line 268: | Line 223: | ||
| 1990-1995 || Scientific development || Concept introduction || The term ''{{w|negligible senescence}}'' is first used by professor [[wikipedia:Caleb Finch|Caleb Finch]] to describe organisms such as [[wikipedia:lobsters|lobsters]] and [[wikipedia:hydras|hydras]], which do not show symptoms of aging.<ref>{{cite book |author=Greg Critser |title=Eternity Soup: Inside the Quest to End Aging |url=https://books.google.com.ar/books?id=5ikIgPLoM7IC&pg=PT173&lpg=PT173&dq=The+term+negligible+senescence+is+first+used+by+professor+Caleb+Finch&source=bl&ots=8x928wNPqZ&sig=tsCM_DxFBQ-43HGdliqI1NsrsAs&hl=es&sa=X&ved=0ahUKEwjR6rLK79DMAhXCQpAKHfDdBKEQ6AEIOjAE#v=onepage&q=The%20term%20negligible%20senescence%20is%20first%20used%20by%20professor%20Caleb%20Finch&f=false |location= |publisher= |page= |date= |isbn=}}</ref> || | | 1990-1995 || Scientific development || Concept introduction || The term ''{{w|negligible senescence}}'' is first used by professor [[wikipedia:Caleb Finch|Caleb Finch]] to describe organisms such as [[wikipedia:lobsters|lobsters]] and [[wikipedia:hydras|hydras]], which do not show symptoms of aging.<ref>{{cite book |author=Greg Critser |title=Eternity Soup: Inside the Quest to End Aging |url=https://books.google.com.ar/books?id=5ikIgPLoM7IC&pg=PT173&lpg=PT173&dq=The+term+negligible+senescence+is+first+used+by+professor+Caleb+Finch&source=bl&ots=8x928wNPqZ&sig=tsCM_DxFBQ-43HGdliqI1NsrsAs&hl=es&sa=X&ved=0ahUKEwjR6rLK79DMAhXCQpAKHfDdBKEQ6AEIOjAE#v=onepage&q=The%20term%20negligible%20senescence%20is%20first%20used%20by%20professor%20Caleb%20Finch&f=false |location= |publisher= |page= |date= |isbn=}}</ref> || | ||
|- | |- | ||
| + | | 1991 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Applying a similar approach by Nesse in 1988 (see row), but to a larger set of wild mammalian populations, Promislow again concludes that there is detectable senescence in wild populations of animals.<ref name="Shefferson"/> || | ||
| + | |- | ||
| 1991 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || {{w|Leonid A. Gavrilov}} and Natalia S. Gavrilova apply the principles of [[wikipedia:reliability theory|reliability theory]] to human biology, proposing a [[wikipedia:Reliability theory of aging and longevity|reliabity theory of aging]] which is based on the premise that humans are born in a highly defective state. According to the model, this is then made worse by environmental and mutational damage, and survival of the organism depends on [[wikipedia:Redundancy (engineering)|redundancy]].<ref>{{cite magazine |author= A. J. S. Rayl |title=Aging, in Theory: A Personal Pursuit |url=http://www.the-scientist.com/?articles.view/articleNo/14015/title/Aging--in-Theory--A-Personal-Pursuit/ |magazine= The Scientist|location= |publisher= |date= May 13, 2002 |access-date= }}</ref><ref>Leonid A. Gavrilov, Natalia S. Gavrilova; V.P. Skulachev (ed.); John and Liliya Payne (trans.) (1991). ''The Biology of Life Span: A Quantitative Approach''. Chur; New York: Harwood Academic Publishers. ISBN 9783718649839.</ref>|| | | 1991 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || {{w|Leonid A. Gavrilov}} and Natalia S. Gavrilova apply the principles of [[wikipedia:reliability theory|reliability theory]] to human biology, proposing a [[wikipedia:Reliability theory of aging and longevity|reliabity theory of aging]] which is based on the premise that humans are born in a highly defective state. According to the model, this is then made worse by environmental and mutational damage, and survival of the organism depends on [[wikipedia:Redundancy (engineering)|redundancy]].<ref>{{cite magazine |author= A. J. S. Rayl |title=Aging, in Theory: A Personal Pursuit |url=http://www.the-scientist.com/?articles.view/articleNo/14015/title/Aging--in-Theory--A-Personal-Pursuit/ |magazine= The Scientist|location= |publisher= |date= May 13, 2002 |access-date= }}</ref><ref>Leonid A. Gavrilov, Natalia S. Gavrilova; V.P. Skulachev (ed.); John and Liliya Payne (trans.) (1991). ''The Biology of Life Span: A Quantitative Approach''. Chur; New York: Harwood Academic Publishers. ISBN 9783718649839.</ref>|| | ||
|- | |- | ||
| − | | 1991 || Scientific development || Laboratory || {{w|Leonard Hayflick}} describes an increase in cell degeneration and debris, resembling cell death.<ref name="Ogrodnik"/> || {{w|United States}} | + | | 1991 || Scientific development || Laboratory research || {{w|Leonard Hayflick}} describes an increase in cell degeneration and debris, resembling cell death.<ref name="Ogrodnik"/> || {{w|United States}} |
|- | |- | ||
| 1992 || Scientific development || Data science || The {{w|National Archive of Computerized Data on Aging}} (NACDA) publishes in the Internet the first 28 datasets related to aging. Gradually, the number of published datasets would surpass 1600. These datasets are available to any researcher around the world at no charge, so they can search in them for new patterns. The site also provides some tools to facilitate analysis.<ref>{{cite web|url=https://www.icpsr.umich.edu/web/pages/NACDA/index.html |title=About Us |work={{abbr|NACDA|National Archive of Computerized Data on Aging}} |access-date=7 May 2021}}</ref> || {{w|United States}} | | 1992 || Scientific development || Data science || The {{w|National Archive of Computerized Data on Aging}} (NACDA) publishes in the Internet the first 28 datasets related to aging. Gradually, the number of published datasets would surpass 1600. These datasets are available to any researcher around the world at no charge, so they can search in them for new patterns. The site also provides some tools to facilitate analysis.<ref>{{cite web|url=https://www.icpsr.umich.edu/web/pages/NACDA/index.html |title=About Us |work={{abbr|NACDA|National Archive of Computerized Data on Aging}} |access-date=7 May 2021}}</ref> || {{w|United States}} | ||
|- | |- | ||
| − | | 1993 || Scientific development || Laboratory || American molecular biologist {{w|Cynthia Kenyon}} discovers that a single-gene mutation ([[wikipedia:Daf-2|Daf-2]]) can double the lifespan of nematode ''{{w|Caenorhabditis elegans}}'' and that this can be reversed by a second mutation in [[wikipedia:daf-16|daf-16]]m.<ref>{{cite web|url=http://www.sfgate.com/magazine/article/Finding-the-Fountain-of-Youth-Where-will-UCSF-2667274.php|title= Finding the Fountain of Youth / Where will UCSF biochemist Cynthia Kenyon's age-bending experiments with worms lead us?}}</ref><ref>{{cite journal |vauthors=Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R | title=A C. elegans mutant that lives twice as long as wild type | journal=[[wikipedia:Nature (journal)|Nature]] | volume=366 | issue=6454 | year=1993 | pages=461–464 | pmid=8247153 | doi=10.1038/366461a0}}</ref> || {{w|United States}} || [[File:Cyntia Kenyon 01.JPG|thumb|center| | + | | 1993 || Scientific development || Laboratory research || American molecular biologist {{w|Cynthia Kenyon}} discovers that a single-gene mutation ([[wikipedia:Daf-2|Daf-2]]) can double the lifespan of nematode ''{{w|Caenorhabditis elegans}}'' and that this can be reversed by a second mutation in [[wikipedia:daf-16|daf-16]]m.<ref>{{cite web|url=http://www.sfgate.com/magazine/article/Finding-the-Fountain-of-Youth-Where-will-UCSF-2667274.php|title= Finding the Fountain of Youth / Where will UCSF biochemist Cynthia Kenyon's age-bending experiments with worms lead us?}}</ref><ref>{{cite journal |vauthors=Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R | title=A C. elegans mutant that lives twice as long as wild type | journal=[[wikipedia:Nature (journal)|Nature]] | volume=366 | issue=6454 | year=1993 | pages=461–464 | pmid=8247153 | doi=10.1038/366461a0}}</ref> || {{w|United States}} || [[File:Cyntia Kenyon 01.JPG|thumb|center|100px|{{w|Cynthia Kenyon}}]] |
|- | |- | ||
| 1994 || Literature || || [[wikipedia:Leonard Hayflick|Leonard Hayflick]] publishes ''How and Why we Age'', which elaborates on the difference between biological and chronological age and then explores on how understanding of aging has changed through history.<ref>{{cite web|url=https://repository.asu.edu/attachments/163946/content/Bartlett_asu_0010N_15494.pdf|title=A History of Cellular Senescence and Its Relation to Stem Cells in the Twentieth and Twenty-First Centuries |author=Zane Bartlett |date=|accessdate=4 August 2016}}</ref> || || | | 1994 || Literature || || [[wikipedia:Leonard Hayflick|Leonard Hayflick]] publishes ''How and Why we Age'', which elaborates on the difference between biological and chronological age and then explores on how understanding of aging has changed through history.<ref>{{cite web|url=https://repository.asu.edu/attachments/163946/content/Bartlett_asu_0010N_15494.pdf|title=A History of Cellular Senescence and Its Relation to Stem Cells in the Twentieth and Twenty-First Centuries |author=Zane Bartlett |date=|accessdate=4 August 2016}}</ref> || || | ||
|- | |- | ||
| − | | 1995 || Scientific development || Laboratory || Detection of {{w|senescent cell}}s using a cytochemical assay is first described.<ref>{{cite web|title=Senescence Associated β-galactosidase Staining|url=http://www.bio-protocol.org/e247|accessdate=20 August 2016}}</ref> Researchers discover that senescent cells express a β-galactosidase activity; and describe the “senescence-associated β galactosidase” (SA-βgal) biomarker, which conveniently identifies individual senescent cells in vitro and in vivo.<ref>{{cite journal |last1=Itahana |first1=Koji |last2=Itahana |first2=Yoko |last3=Dimri |first3=Goberdhan P. |title=Colorimetric Detection of Senescence-Associated β Galactosidase |journal=Methods in molecular biology (Clifton, N.J.) |date=2013 |volume=965 |pages=143–156 |doi=10.1007/978-1-62703-239-1_8 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3769963/ |issn=1064-3745}}</ref> || | + | | 1994 || Scientific development || Laboratory research || Frisch and Francis report that loss of matrix attachment results in apoptosis. Since then, studies aimed at understanding the molecular mechanisms underlying the phenomenon coupled with advances in the field of apoptosis overall would help to elucidate several of the key players and to create hypotheses for how they are regulated.<ref name="Gewirtz"/> || |
| + | |- | ||
| + | | 1995 || Scientific development || Laboratory research || Detection of {{w|senescent cell}}s using a cytochemical assay is first described.<ref>{{cite web|title=Senescence Associated β-galactosidase Staining|url=http://www.bio-protocol.org/e247|accessdate=20 August 2016}}</ref> Researchers discover that senescent cells express a β-galactosidase activity; and describe the “senescence-associated β galactosidase” (SA-βgal) biomarker, which conveniently identifies individual senescent cells in vitro and in vivo.<ref>{{cite journal |last1=Itahana |first1=Koji |last2=Itahana |first2=Yoko |last3=Dimri |first3=Goberdhan P. |title=Colorimetric Detection of Senescence-Associated β Galactosidase |journal=Methods in molecular biology (Clifton, N.J.) |date=2013 |volume=965 |pages=143–156 |doi=10.1007/978-1-62703-239-1_8 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3769963/ |issn=1064-3745}}</ref> || | ||
|- | |- | ||
| − | | 1995 || Scientific development || Laboratory || The existence of {{w|senescence-associated beta-galactosidase}} is proposed by Dimri et al.<ref name="pmid7568133">{{cite journal |vauthors=Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O | title = A biomarker that identifies senescent human cells in culture and in aging skin in vivo | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 92 | issue = 20 | pages = 9363–7 |date=September 1995 | pmid = 7568133 | pmc = 40985 | doi = 10.1073/pnas.92.20.9363}}</ref> following the observation that when {{w|beta-galactosidase}} assays were carried out at pH 6.0, only cells in {{w|senescence}} state develop staining. They proposed a cytochemical assay based on production of a blue-dyed precipitate that results from the cleavage of the chromogenic substrate {{w|X-Gal}}, which stains blue when cleaved by galactosidase. Since then, even more specific quantitative assays were developed for its detection at pH 6.0.<ref name="pmid17920029">{{cite journal |vauthors=Bassaneze V, Miyakawa AA, Krieger JE | title = A quantitative chemiluminescent method for studying replicative and stress-induced premature senescence in cell cultures | journal = Anal. Biochem. | volume = 372 | issue = 2 | pages = 198–203 |date=January 2008 | pmid = 17920029 | doi = 10.1016/j.ab.2007.08.016 }}</ref><ref name="pmid16004951">{{cite journal |vauthors=Gary RK, Kindell SM | title = Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts | journal = Anal. Biochem. | volume = 343 | issue = 2 | pages = 329–34 |date=August 2005 | pmid = 16004951 | doi = 10.1016/j.ab.2005.06.003 }}</ref><ref name="pmid17634571">{{cite book | vauthors = Itahana K, Campisi J, Dimri GP | title = Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay | volume = 371 | pages = [https://archive.org/details/biologicalagingm00toll/page/21 21–31] | year = 2007 | pmid = 17634571 | doi = 10.1007/978-1-59745-361-5_3 | url = https://archive.org/details/biologicalagingm00toll/page/21 | series = Methods in Molecular Biology | isbn = 978-1-58829-658-0 | url-access = registration }}</ref> || | + | | 1995 || Scientific development || Laboratory research || The existence of {{w|senescence-associated beta-galactosidase}} is proposed by Dimri et al.<ref name="pmid7568133">{{cite journal |vauthors=Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O | title = A biomarker that identifies senescent human cells in culture and in aging skin in vivo | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 92 | issue = 20 | pages = 9363–7 |date=September 1995 | pmid = 7568133 | pmc = 40985 | doi = 10.1073/pnas.92.20.9363}}</ref> following the observation that when {{w|beta-galactosidase}} assays were carried out at pH 6.0, only cells in {{w|senescence}} state develop staining. They proposed a cytochemical assay based on production of a blue-dyed precipitate that results from the cleavage of the chromogenic substrate {{w|X-Gal}}, which stains blue when cleaved by galactosidase. Since then, even more specific quantitative assays were developed for its detection at pH 6.0.<ref name="pmid17920029">{{cite journal |vauthors=Bassaneze V, Miyakawa AA, Krieger JE | title = A quantitative chemiluminescent method for studying replicative and stress-induced premature senescence in cell cultures | journal = Anal. Biochem. | volume = 372 | issue = 2 | pages = 198–203 |date=January 2008 | pmid = 17920029 | doi = 10.1016/j.ab.2007.08.016 }}</ref><ref name="pmid16004951">{{cite journal |vauthors=Gary RK, Kindell SM | title = Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts | journal = Anal. Biochem. | volume = 343 | issue = 2 | pages = 329–34 |date=August 2005 | pmid = 16004951 | doi = 10.1016/j.ab.2005.06.003 }}</ref><ref name="pmid17634571">{{cite book | vauthors = Itahana K, Campisi J, Dimri GP | title = Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay | volume = 371 | pages = [https://archive.org/details/biologicalagingm00toll/page/21 21–31] | year = 2007 | pmid = 17634571 | doi = 10.1007/978-1-59745-361-5_3 | url = https://archive.org/details/biologicalagingm00toll/page/21 | series = Methods in Molecular Biology | isbn = 978-1-58829-658-0 | url-access = registration }}</ref> || |
|- | |- | ||
| 1995 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The remodeling theory of aging is introduced.<ref name="Yashin"/> It postulates that immunosenescence or 'immuno-remodelling' is a dynamic process involving both loss and gains of immune function.<ref>{{cite journal |last1=Franceschi |first1=C |last2=Valensin |first2=S |last3=Bonafè |first3=M |last4=Paolisso |first4=G |last5=Yashin |first5=A.I |last6=Monti |first6=D |last7=De Benedictis |first7=G |title=The network and the remodeling theories of aging: historical background and new perspectives |journal=Experimental Gerontology |date=September 2000 |volume=35 |issue=6-7 |pages=879–896 |doi=10.1016/S0531-5565(00)00172-8}}</ref> || | | 1995 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || The remodeling theory of aging is introduced.<ref name="Yashin"/> It postulates that immunosenescence or 'immuno-remodelling' is a dynamic process involving both loss and gains of immune function.<ref>{{cite journal |last1=Franceschi |first1=C |last2=Valensin |first2=S |last3=Bonafè |first3=M |last4=Paolisso |first4=G |last5=Yashin |first5=A.I |last6=Monti |first6=D |last7=De Benedictis |first7=G |title=The network and the remodeling theories of aging: historical background and new perspectives |journal=Experimental Gerontology |date=September 2000 |volume=35 |issue=6-7 |pages=879–896 |doi=10.1016/S0531-5565(00)00172-8}}</ref> || | ||
|- | |- | ||
| − | | 1995 || Scientific development || Laboratory || Research by by E. Wang shows that cellular senescence is associated with a reduced sensitivity to {{w|cell death}}.<ref name="Ogrodnik"/><ref>{{cite journal |last1=Wang |first1=E. |title=Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved |journal=Cancer Research |date=1995-06-01 |volume=55 |issue=11 |pages=2284–2292 |url=https://pubmed.ncbi.nlm.nih.gov/7757977/ |issn=0008-5472}}</ref> || || | + | | 1995 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Slade suggests that actuarial senescence may be negligible in small mammals.<ref name="Shefferson"/> || |
| + | |- | ||
| + | | 1995 || Scientific development || Laboratory research || Research by by E. Wang shows that cellular senescence is associated with a reduced sensitivity to {{w|cell death}}.<ref name="Ogrodnik"/><ref>{{cite journal |last1=Wang |first1=E. |title=Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved |journal=Cancer Research |date=1995-06-01 |volume=55 |issue=11 |pages=2284–2292 |url=https://pubmed.ncbi.nlm.nih.gov/7757977/ |issn=0008-5472}}</ref> || || | ||
|- | |- | ||
| − | | | + | | 1998 || Scientific development || Laboratory research || Scientists manage to extend, in a laboratory environment, the life of [[w:Somatic cell|normal]] human cells beyond the {{w|Hayflick limit}} using {{w|telomerase}}.<ref>{{cite journal |last1=Varela |first1=E |last2=Blasco |first2=M A |title=2009 Nobel Prize in Physiology or Medicine: telomeres and telomerase |journal=Oncogene |date=March 2010 |volume=29 |issue=11 |pages=1561–1565 |doi=10.1038/onc.2010.15}}</ref><ref>{{cite journal |last1=Bodnar |first1=A. G. |title=Extension of Life-Span by Introduction of Telomerase into Normal Human Cells |journal=Science |date=16 January 1998 |volume=279 |issue=5349 |pages=349–352 |doi=10.1126/science.279.5349.349}}</ref> || |
|- | |- | ||
| − | | 1998 || Scientific development || | + | | 1998 || Scientific development || || Martinez observes that ''[[w:Hydra (genus)|Hydra]]'' appear to grow older without showing signs of senescence. This is entirely consistent with the principle that the evolution of disposability of the soma presupposes the existence of a clear germ–soma distinction.<ref name="Shefferson"/> || |
|- | |- | ||
| − | | | + | | 1998 || Scientific development || || American academic {{w|Caleb Finch}}, and later other gerontologists and some demographers, argue that there is evidence of negligible senescence in natural populations of a number of species, from rockfish to tortoises.<ref name="Shefferson"/> || {{w|United States}} |
|- | |- | ||
| − | | 1999 || Literature || || {{w|Aubrey de Grey}} publishes ''The Mitochondrial Free Radical Theory of Aging'', which introduces the term "engineered negligible senescence".<ref name="isbn1587061554">de Grey, Aubrey (November 2003). ''The Mitochondrial Free Radical Theory of Aging.'' Austin, Texas: Landes Bioscience.</ref> || || | + | | 1999 || Organization (research institute) || || The {{w|Buck Institute for Research on Aging}} is established as an independent biomedical research institute devoted solely to research on aging and age-related diseases.<ref>{{cite web |title=About |url=https://www.buckinstitute.org/about/ |website=BUCK |access-date=9 June 2021}}</ref><ref>{{cite web |title=Buck Institute for Research on Aging |url=https://www.omicsonline.org/universities/Buck_Institute_for_Research_on_Aging/ |website=www.omicsonline.org |access-date=9 June 2021}}</ref> || {{w|United States}} || [[File:Buck logo tagline period.png|thumb|center|100px]] |
| + | |- | ||
| + | | 1999 || Literature || || {{w|Aubrey de Grey}} publishes ''The Mitochondrial Free Radical Theory of Aging'', which introduces the term "engineered negligible senescence".<ref name="isbn1587061554">de Grey, Aubrey (November 2003). ''The Mitochondrial Free Radical Theory of Aging.'' Austin, Texas: Landes Bioscience.</ref> || || [[File:Aubrey de Grey.jpg|thumb|center|100px|{{w|Aubrey de Grey}}]] | ||
|- | |- | ||
| 2000 || Scienific development || Concept introduction || Sabina Sperandio and colleagues introduce the term "{{w|paraptosis}}". The group uses human [[w:Insulin-like growth factor 1 receptor|insulin-like growth factor 1 receptor (IGF-1R)]] to stimulate cell death in [[w:HEK 293 cells|293T cells]] and mouse embryonic {{w|fibroblast}}s, observing distinct differences from other forms of cell death. They coin the term "paraptosis", derived from the Greek preposition ''para'', meaning beside or related to, and ''{{w|apoptosis}}''.<ref name="Sperandio (2000)">{{cite journal|last=Sperandio|first=S |author2=de Belle, I |author3=Bredesen, DE|title=An alternative, nonapoptotic form of programmed cell death.|journal=Proceedings of the National Academy of Sciences of the United States of America|date=Dec 19, 2000|volume=97|issue=26|pages=14376–81|pmid=11121041|doi=10.1073/pnas.97.26.14376|pmc=18926}}</ref><ref>{{cite web|title=Paraptosis - Oxford Dictionaries|url=http://www.oxforddictionaries.com/definition/english/paraptosis}}</ref> || {{w|United States}} | | 2000 || Scienific development || Concept introduction || Sabina Sperandio and colleagues introduce the term "{{w|paraptosis}}". The group uses human [[w:Insulin-like growth factor 1 receptor|insulin-like growth factor 1 receptor (IGF-1R)]] to stimulate cell death in [[w:HEK 293 cells|293T cells]] and mouse embryonic {{w|fibroblast}}s, observing distinct differences from other forms of cell death. They coin the term "paraptosis", derived from the Greek preposition ''para'', meaning beside or related to, and ''{{w|apoptosis}}''.<ref name="Sperandio (2000)">{{cite journal|last=Sperandio|first=S |author2=de Belle, I |author3=Bredesen, DE|title=An alternative, nonapoptotic form of programmed cell death.|journal=Proceedings of the National Academy of Sciences of the United States of America|date=Dec 19, 2000|volume=97|issue=26|pages=14376–81|pmid=11121041|doi=10.1073/pnas.97.26.14376|pmc=18926}}</ref><ref>{{cite web|title=Paraptosis - Oxford Dictionaries|url=http://www.oxforddictionaries.com/definition/english/paraptosis}}</ref> || {{w|United States}} | ||
|- | |- | ||
| − | | 2003 || Scientific development || Laboratory || Scientists report first evidence that aging of {{w|nematode}}s is regulated via mechanistic target of{{w|rapamycin}} ({{w|mTOR}}) signaling.<ref name="Zainabadi">{{cite journal |last1=Zainabadi |first1=Kayvan |title=A brief history of modern aging research |journal=Experimental Gerontology |date=April 2018 |volume=104 |pages=35–42 |doi=10.1016/j.exger.2018.01.018}}</ref><ref>{{cite journal| vauthors = McCay CM, Crowell M |date=October 1934 |title=Prolonging the Life Span |url=https://www.jstor.org/stable/15813 |journal={{w|The Scientific Monthly}} |volume=39 |issue=5 |pages=405–414 |doi= |jstor=15813 |pmc= |pmid= }}</ref><ref>{{cite journal |last1=Vellai |first1=Tibor |last2=Takacs-Vellai |first2=Krisztina |last3=Zhang |first3=Yue |last4=Kovacs |first4=Attila L. |last5=Orosz |first5=László |last6=Müller |first6=Fritz |title=Influence of TOR kinase on lifespan in C. elegans |journal=Nature |date=December 2003 |volume=426 |issue=6967 |pages=620–620 |doi=10.1038/426620a}}</ref> || | + | | 2003 || Scientific development || Laboratory research || Scientists report first evidence that aging of {{w|nematode}}s is regulated via mechanistic target of{{w|rapamycin}} ({{w|mTOR}}) signaling.<ref name="Zainabadi">{{cite journal |last1=Zainabadi |first1=Kayvan |title=A brief history of modern aging research |journal=Experimental Gerontology |date=April 2018 |volume=104 |pages=35–42 |doi=10.1016/j.exger.2018.01.018}}</ref><ref>{{cite journal| vauthors = McCay CM, Crowell M |date=October 1934 |title=Prolonging the Life Span |url=https://www.jstor.org/stable/15813 |journal={{w|The Scientific Monthly}} |volume=39 |issue=5 |pages=405–414 |doi= |jstor=15813 |pmc= |pmid= }}</ref><ref>{{cite journal |last1=Vellai |first1=Tibor |last2=Takacs-Vellai |first2=Krisztina |last3=Zhang |first3=Yue |last4=Kovacs |first4=Attila L. |last5=Orosz |first5=László |last6=Müller |first6=Fritz |title=Influence of TOR kinase on lifespan in C. elegans |journal=Nature |date=December 2003 |volume=426 |issue=6967 |pages=620–620 |doi=10.1038/426620a}}</ref> || |
|- | |- | ||
| − | | 2003 || Scientific development || Laboratory || Andrzej Bartke creates a mouse that lived 1819 days (5 years without 7 days), while the maximum lifespan for this species is 1030–1070 days.<ref name="medi">{{cite web|author=В.Е. Чернилевский, В.Н. Крутько |url=https://medi.ru/info/10323/ |title=История изучения средств продления жизни |language=ru |trans-title=History of studying the means of extending life |publisher=National Gerontology Center (of Russia) |date=2000 |access-date=5 May 2021}}</ref> Translated to human standards, such longevity is equivalent to about 180 years.<ref>{{cite web |title=Battle for 'old mouse' prize |url=http://news.bbc.co.uk/2/hi/uk_news/england/cambridgeshire/3079834.stm |website=news.bbc.co.uk |access-date=9 July 2021 |date=4 September 2003}}</ref> || | + | | 2003 || Scientific development || Laboratory research || Andrzej Bartke creates a mouse that lived 1819 days (5 years without 7 days), while the maximum lifespan for this species is 1030–1070 days.<ref name="medi">{{cite web|author=В.Е. Чернилевский, В.Н. Крутько |url=https://medi.ru/info/10323/ |title=История изучения средств продления жизни |language=ru |trans-title=History of studying the means of extending life |publisher=National Gerontology Center (of Russia) |date=2000 |access-date=5 May 2021}}</ref> Translated to human standards, such longevity is equivalent to about 180 years.<ref>{{cite web |title=Battle for 'old mouse' prize |url=http://news.bbc.co.uk/2/hi/uk_news/england/cambridgeshire/3079834.stm |website=news.bbc.co.uk |access-date=9 July 2021 |date=4 September 2003}}</ref> || |
| + | |- | ||
| + | | 2004 || Scientific development || || Vaupel et al. describe two cases: one being a species that escapes from senescence which would exhibit the lack of such decline, referred to as "negligible senescence", and a second case in which a species shows even physiological improvement with age, which may be referred to as "negative senescence".<ref name="Shefferson"/> || | ||
|- | |- | ||
| 2004 || Scientific development || {{w|Data science}} || GenAge launches as the first curated [[w:biological database|database]] of genes related to human ageing.<ref>{{cite journal |last1=Magalhães |first1=João Pedro de |last2=Toussaint |first2=Olivier |title=GenAge: a genomic and proteomic network map of human ageing |journal=FEBS Letters |date=2004 |volume=571 |issue=1–3 |pages=243–247 |doi=10.1016/j.febslet.2004.07.006 |url=https://febs.onlinelibrary.wiley.com/doi/full/10.1016/j.febslet.2004.07.006 |access-date=26 June 2021 |language=en |issn=1873-3468|doi-access=free }}</ref> It stores data of genes commonly altered during ageing, drawn from a microarray meta-analysis study, and the LongevityMap, a database of human genetic variants associated with longevity.<ref>{{cite web |title=GenAge: The Ageing Gene Database |url=https://genomics.senescence.info/genes/index.html |website=genomics.senescence.info |access-date=17 August 2021}}</ref> || | | 2004 || Scientific development || {{w|Data science}} || GenAge launches as the first curated [[w:biological database|database]] of genes related to human ageing.<ref>{{cite journal |last1=Magalhães |first1=João Pedro de |last2=Toussaint |first2=Olivier |title=GenAge: a genomic and proteomic network map of human ageing |journal=FEBS Letters |date=2004 |volume=571 |issue=1–3 |pages=243–247 |doi=10.1016/j.febslet.2004.07.006 |url=https://febs.onlinelibrary.wiley.com/doi/full/10.1016/j.febslet.2004.07.006 |access-date=26 June 2021 |language=en |issn=1873-3468|doi-access=free }}</ref> It stores data of genes commonly altered during ageing, drawn from a microarray meta-analysis study, and the LongevityMap, a database of human genetic variants associated with longevity.<ref>{{cite web |title=GenAge: The Ageing Gene Database |url=https://genomics.senescence.info/genes/index.html |website=genomics.senescence.info |access-date=17 August 2021}}</ref> || | ||
| + | |- | ||
| + | | 2004 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Reznick et al. suggest the need to develop ecologically oriented "derived" theories of ageing that include density- and condition-dependent interactions with senescent mortality. They state that density dependence affects aging because it interacts with the age profile of mortality and can lead to changes in resource availability. Also, like density dependence, they state that condition dependence can interact with extrinsic mortality and the rate of aging.<ref name="Shefferson"/> || | ||
| + | |- | ||
| + | | 2005 || Scientific development || || Kirkwood discovers that unicellular organisms such as yeasts and bacteria can exhibit forms of senescence.<ref name="Shefferson"/> || | ||
|- | |- | ||
| 2006 || Organization (research network) || || {{w|Network Aging Research}} (Netzwerk Alternsforschung) is founded in {{w|Germany}} at {{w|Heidelberg University}} as a research network on the topic of aging. It supports researchers in multiple fields of aging, including humanists, natural- and medical scientists.<ref>{{cite web |title=Das Netzwerk AlternsfoRschung (NAR) der Ruprecht-Karls-Universität Heidelberg (PDF-E-Book). Psychotherapie im Alter 2010, 7(3), 405-410 |url=https://www.psychosozial-verlag.de/20255 |website=Psychosozial-Verlag |access-date=9 June 2021 |language=de}}</ref> || {{w|Germany}} | | 2006 || Organization (research network) || || {{w|Network Aging Research}} (Netzwerk Alternsforschung) is founded in {{w|Germany}} at {{w|Heidelberg University}} as a research network on the topic of aging. It supports researchers in multiple fields of aging, including humanists, natural- and medical scientists.<ref>{{cite web |title=Das Netzwerk AlternsfoRschung (NAR) der Ruprecht-Karls-Universität Heidelberg (PDF-E-Book). Psychotherapie im Alter 2010, 7(3), 405-410 |url=https://www.psychosozial-verlag.de/20255 |website=Psychosozial-Verlag |access-date=9 June 2021 |language=de}}</ref> || {{w|Germany}} | ||
| Line 306: | Line 275: | ||
| 2006 || Literature (journal) || Clinical study || Peer-reviewed open access medical journal ''{{w|Clinical Interventions in Aging}}'' is founded, covering research in gerontology.<ref>{{cite web |last1=Press |first1=Dove |title=Clinical Interventions in Aging - Dove Press Open Access Publisher |url=https://www.dovepress.com/clinical-interventions-in-aging-journal |website=www.dovepress.com |access-date=2 July 2021}}</ref> || | | 2006 || Literature (journal) || Clinical study || Peer-reviewed open access medical journal ''{{w|Clinical Interventions in Aging}}'' is founded, covering research in gerontology.<ref>{{cite web |last1=Press |first1=Dove |title=Clinical Interventions in Aging - Dove Press Open Access Publisher |url=https://www.dovepress.com/clinical-interventions-in-aging-journal |website=www.dovepress.com |access-date=2 July 2021}}</ref> || | ||
|- | |- | ||
| − | | 2006 || Scientific development || Laboratory || {{w|Shinya Yamanaka}} and {{w|John Gurdon}} receive the {{w|Nobel Prize in Physiology or Medicine}} for their work on reprogramming mature cells into pluripotent cells.<ref>{{cite web |title=The Nobel Prize in Physiology or Medicine 2012 |url=https://www.nobelprize.org/prizes/medicine/2012/summary/ |website=NobelPrize.org |access-date=9 July 2021}}</ref> Yamanaka is the first to produce {{w|induced stem cells}} (iSC) from somatic cells by the simultaneous action of several factors.<ref>{{cite journal |last1=Takahashi |first1=Kazutoshi |last2=Yamanaka |first2=Shinya |title=Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors |journal=Cell |date=August 2006 |volume=126 |issue=4 |pages=663–676 |doi=10.1016/j.cell.2006.07.024}}</ref><ref>{{cite journal |last1=Takahashi |first1=Kazutoshi |last2=Tanabe |first2=Koji |last3=Ohnuki |first3=Mari |last4=Narita |first4=Megumi |last5=Ichisaka |first5=Tomoko |last6=Tomoda |first6=Kiichiro |last7=Yamanaka |first7=Shinya |title=Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors |journal=Cell |date=November 2007 |volume=131 |issue=5 |pages=861–872 |doi=10.1016/j.cell.2007.11.019}}</ref><ref>{{cite journal |last1=Okita |first1=Keisuke |last2=Ichisaka |first2=Tomoko |last3=Yamanaka |first3=Shinya |title=Generation of germline-competent induced pluripotent stem cells |journal=Nature |date=July 2007 |volume=448 |issue=7151 |pages=313–317 |doi=10.1038/nature05934}}</ref> | + | | 2006 || Scientific development || Laboratory research || {{w|Shinya Yamanaka}} and {{w|John Gurdon}} receive the {{w|Nobel Prize in Physiology or Medicine}} for their work on reprogramming mature cells into pluripotent cells.<ref>{{cite web |title=The Nobel Prize in Physiology or Medicine 2012 |url=https://www.nobelprize.org/prizes/medicine/2012/summary/ |website=NobelPrize.org |access-date=9 July 2021}}</ref> Yamanaka is the first to produce {{w|induced stem cells}} (iSC) from somatic cells by the simultaneous action of several factors.<ref>{{cite journal |last1=Takahashi |first1=Kazutoshi |last2=Yamanaka |first2=Shinya |title=Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors |journal=Cell |date=August 2006 |volume=126 |issue=4 |pages=663–676 |doi=10.1016/j.cell.2006.07.024}}</ref><ref>{{cite journal |last1=Takahashi |first1=Kazutoshi |last2=Tanabe |first2=Koji |last3=Ohnuki |first3=Mari |last4=Narita |first4=Megumi |last5=Ichisaka |first5=Tomoko |last6=Tomoda |first6=Kiichiro |last7=Yamanaka |first7=Shinya |title=Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors |journal=Cell |date=November 2007 |volume=131 |issue=5 |pages=861–872 |doi=10.1016/j.cell.2007.11.019}}</ref><ref>{{cite journal |last1=Okita |first1=Keisuke |last2=Ichisaka |first2=Tomoko |last3=Yamanaka |first3=Shinya |title=Generation of germline-competent induced pluripotent stem cells |journal=Nature |date=July 2007 |volume=448 |issue=7151 |pages=313–317 |doi=10.1038/nature05934}}</ref> || || [[File:Shinya yamanaka10.jpg|thumb|center|100px|{{w|Shinya Yamanaka}}]] |
|- | |- | ||
| − | | 2007 || Scientific development || Laboratory || Researchers manage to extend mouse lifespan via deletion of {{w|insulin}} receptor in the brain.<ref name="Zainabadi" /><ref>{{cite journal |last1=Taguchi |first1=A. |last2=Wartschow |first2=L. M. |last3=White |first3=M. F. |title=Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis |journal=Science |date=20 July 2007 |volume=317 |issue=5836 |pages=369–372 |doi=10.1126/science.1142179}}</ref> || | + | | 2007 || Scientific development || Laboratory research || Researchers manage to extend mouse lifespan via deletion of {{w|insulin}} receptor in the brain.<ref name="Zainabadi" /><ref>{{cite journal |last1=Taguchi |first1=A. |last2=Wartschow |first2=L. M. |last3=White |first3=M. F. |title=Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis |journal=Science |date=20 July 2007 |volume=317 |issue=5836 |pages=369–372 |doi=10.1126/science.1142179}}</ref> || |
|- | |- | ||
| − | | 2007 || Scientific development || Laboratory || Researchers report first evidence that a {{w|pharmacological agent}} (namely, {{w|metformin}}) at a certain dosage is capable to increase the lifespan of mice.<ref name="Zainabadi" /><ref>{{cite journal |last1=Anisimov |first1=Vladimir N. |last2=Berstein |first2=Lev M. |last3=Egormin |first3=Peter A. |last4=Piskunova |first4=Tatiana S. |last5=Popovich |first5=Irina G. |last6=Zabezhinski |first6=Mark A. |last7=Tyndyk |first7=Margarita L. |last8=Yurova |first8=Maria V. |last9=Kovalenko |first9=Irina G. |last10=Poroshina |first10=Tatiana E. |last11=Semenchenko |first11=Anna V. |title=Metformin slows down aging and extends life span of female SHR mice |journal=Cell Cycle |date=September 2008 |volume=7 |issue=17 |pages=2769–2773 |doi=10.4161/cc.7.17.6625}}</ref> || | + | | 2007 || Scientific development || || Study by Passos, Saretzki, and Ahmed et al. indicates that mitochondrial-derived reactive oxygen species can generate DNA damage, including single-stranded breaks in telomere regions, contributing to accelerated telomere shortening and premature senescence.<ref name="New York, N"/><ref>{{cite journal |last1=Passos |first1=João F |last2=Saretzki |first2=Gabriele |last3=Ahmed |first3=Shaheda |last4=Nelson |first4=Glyn |last5=Richter |first5=Torsten |last6=Peters |first6=Heiko |last7=Wappler |first7=Ilka |last8=Birket |first8=Matthew J |last9=Harold |first9=Graham |last10=Schaeuble |first10=Karin |last11=Birch-Machin |first11=Mark A |last12=Kirkwood |first12=Thomas B. L |last13=von Zglinicki |first13=Thomas |title=Mitochondrial Dysfunction Accounts for the Stochastic Heterogeneity in Telomere-Dependent Senescence |journal=PLoS Biology |date=1 May 2007 |volume=5 |issue=5 |pages=e110 |doi=10.1371/journal.pbio.0050110}}</ref> || {{w|United Kingdom}} ({{w|University of Newcastle}}) |
| + | |- | ||
| + | | 2007 || Scientific development || Laboratory research || Researchers report first evidence that a {{w|pharmacological agent}} (namely, {{w|metformin}}) at a certain dosage is capable to increase the lifespan of mice.<ref name="Zainabadi" /><ref>{{cite journal |last1=Anisimov |first1=Vladimir N. |last2=Berstein |first2=Lev M. |last3=Egormin |first3=Peter A. |last4=Piskunova |first4=Tatiana S. |last5=Popovich |first5=Irina G. |last6=Zabezhinski |first6=Mark A. |last7=Tyndyk |first7=Margarita L. |last8=Yurova |first8=Maria V. |last9=Kovalenko |first9=Irina G. |last10=Poroshina |first10=Tatiana E. |last11=Semenchenko |first11=Anna V. |title=Metformin slows down aging and extends life span of female SHR mice |journal=Cell Cycle |date=September 2008 |volume=7 |issue=17 |pages=2769–2773 |doi=10.4161/cc.7.17.6625}}</ref> || | ||
|- | |- | ||
| 2008 || Scientific development || Concept introduction || The concept of {{w|senescence-associated secretory phenotype}} (SASP) is first established by {{w|Judith Campisi}} and her group, who first publish on the subject.<ref name="pmid19053174">{{cite journal | vauthors=Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J | title=Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor | journal={{w|PLOS Biology}} | volume=6 | issue=12 | pages=2853–2868 | year=2008 | doi = 10.1371/journal.pbio.0060301 | pmc=2592359 | pmid=19053174 }}</ref> || {{w|United States}} | | 2008 || Scientific development || Concept introduction || The concept of {{w|senescence-associated secretory phenotype}} (SASP) is first established by {{w|Judith Campisi}} and her group, who first publish on the subject.<ref name="pmid19053174">{{cite journal | vauthors=Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J | title=Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor | journal={{w|PLOS Biology}} | volume=6 | issue=12 | pages=2853–2868 | year=2008 | doi = 10.1371/journal.pbio.0060301 | pmc=2592359 | pmid=19053174 }}</ref> || {{w|United States}} | ||
| + | |- | ||
| + | | 2008 || Scientific development || || Coppe, Patil, Rodier et al. determine that senescent cells are known to secrete a number of proinflammatory molecules, a phenotype commonly known as the senescence-associated secretory phenotype (SASP).<ref name="New York, N"/><ref>{{cite journal |last1=Coppé |first1=Jean-Philippe |last2=Patil |first2=Christopher K |last3=Rodier |first3=Francis |last4=Sun |first4=Yu |last5=Muñoz |first5=Denise P |last6=Goldstein |first6=Joshua |last7=Nelson |first7=Peter S |last8=Desprez |first8=Pierre-Yves |last9=Campisi |first9=Judith |title=Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor |journal=PLoS Biology |date=2 December 2008 |volume=6 |issue=12 |pages=e301 |doi=10.1371/journal.pbio.0060301}}</ref> || {{w|United States}} | ||
|- | |- | ||
| 2008 || Organization || || The {{w|Max Planck Institute for Biology of Ageing}} is founded. Its overall research aim is to obtain fundamental insights into the [[w:Senescence|aging process]] and thus to pave the way towards healthier aging in humans.<ref>{{cite web |title=Ageing research cluster in Cologne moves closer together |url=https://www.mpg.de/7575444/mpi-biology-ageing-research_building |website=www.mpg.de |access-date=12 July 2021 |language=en}}</ref> || {{w|Germany}} | | 2008 || Organization || || The {{w|Max Planck Institute for Biology of Ageing}} is founded. Its overall research aim is to obtain fundamental insights into the [[w:Senescence|aging process]] and thus to pave the way towards healthier aging in humans.<ref>{{cite web |title=Ageing research cluster in Cologne moves closer together |url=https://www.mpg.de/7575444/mpi-biology-ageing-research_building |website=www.mpg.de |access-date=12 July 2021 |language=en}}</ref> || {{w|Germany}} | ||
|- | |- | ||
| − | | 2009 || Scientific development || Laboratory || A group of three laboratories initiate a study of the effects of {{w|rapamycin}} on the life span of mice. After administering the agent at late ages, the team discovers a significant increase in maximum life span.<ref name="The biol">{{cite journal |last1=Martin |first1=George M. |title=The biology of aging: 1985–2010 and beyond |journal=The FASEB Journal |date=November 2011 |volume=25 |issue=11 |pages=3756–3762 |doi=10.1096/fj.11-1102.ufm |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4050331/ |issn=0892-6638}}</ref> || || | + | | 2008 || Scientific development || || Ricklefs, comparing age-related mortality patterns in birds in captivity and wild populations finds that the rate of senescence is similar to that in wild populations despite the absence of exogenous forces of mortality (i.e. predation, disease) in captivity.<ref name="Shefferson"/> || |
| + | |- | ||
| + | | 2009 || Scientific development || Laboratory research || A group of three laboratories initiate a study of the effects of {{w|rapamycin}} on the life span of mice. After administering the agent at late ages, the team discovers a significant increase in maximum life span.<ref name="The biol">{{cite journal |last1=Martin |first1=George M. |title=The biology of aging: 1985–2010 and beyond |journal=The FASEB Journal |date=November 2011 |volume=25 |issue=11 |pages=3756–3762 |doi=10.1096/fj.11-1102.ufm |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4050331/ |issn=0892-6638}}</ref> || || | ||
|- | |- | ||
| − | | 2009 || Recognition || Laboratory || {{w|Elizabeth Blackburn}}, {{w|Carol Greider}}, and {{w|Jack Szostak}} are awarded the {{w|Nobel Prize in Physiology or Medicine}} "for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase."<ref>{{cite web |title=The Nobel Prize in Physiology or Medicine 2009 |url=https://www.nobelprize.org/prizes/medicine/2009/blackburn/facts/ |website=NobelPrize.org |access-date=16 June 2021}}</ref> These researchers are aware of the implications of their research on telomerase for the biology of aging, in particular {{w|Carol Greider}} who would become quite active in that field.<ref name="The biol"/> || || [[File:Nobel Prize 2009-Press Conference Physiology or Medicine-04.jpg|thumb|center|210px]] | + | | 2009 || Recognition || Laboratory research || {{w|Elizabeth Blackburn}}, {{w|Carol Greider}}, and {{w|Jack Szostak}} are awarded the {{w|Nobel Prize in Physiology or Medicine}} "for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase."<ref>{{cite web |title=The Nobel Prize in Physiology or Medicine 2009 |url=https://www.nobelprize.org/prizes/medicine/2009/blackburn/facts/ |website=NobelPrize.org |access-date=16 June 2021}}</ref> These researchers are aware of the implications of their research on telomerase for the biology of aging, in particular {{w|Carol Greider}} who would become quite active in that field.<ref name="The biol"/> || || [[File:Nobel Prize 2009-Press Conference Physiology or Medicine-04.jpg|thumb|center|210px]] |
|- | |- | ||
| − | | 2009 || Scientific development || Laboratory || Researchers report association of genetic variants in {{w|insulin}}/[[w:Insulin-like growth factor 1|IGF1]] signaling with human longevity.<ref name="Zainabadi" /><ref>{{cite journal | vauthors = Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E | display-authors = 6 | title = Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity | journal = Aging Cell | volume = 8 | issue = 4 | pages = 460–72 | date = August 2009 | pmid = 19489743 | pmc = 3652804 | doi = 10.1111/j.1474-9726.2009.00493.x }}</ref> || | + | | 2009 || Scientific development || Laboratory research || Researchers report association of genetic variants in {{w|insulin}}/[[w:Insulin-like growth factor 1|IGF1]] signaling with human longevity.<ref name="Zainabadi" /><ref>{{cite journal | vauthors = Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E | display-authors = 6 | title = Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity | journal = Aging Cell | volume = 8 | issue = 4 | pages = 460–72 | date = August 2009 | pmid = 19489743 | pmc = 3652804 | doi = 10.1111/j.1474-9726.2009.00493.x }}</ref> || |
| + | |- | ||
| + | | 2010 || Scientific development || Laboratory research || [[wikipedia:Harvard university|Harvard]] scientists reverse aging process in mice through reactivation of [[wikipedia:telomerase|telomerase]].<ref>{{cite web|url=https://www.theguardian.com/science/2010/nov/28/scientists-reverse-aging-mice-humans |title=Harvard scientists reverse the aging process in mice – now for humans }}</ref>|| {{w|United States}} | ||
|- | |- | ||
| − | | 2010 || Scientific development || Laboratory || | + | | 2010 || Scientific development || Laboratory research || Study by Passos, Nelson, Wang, et al. reports that reactive oxygen species produced by senescent cells are shown to lock senescent cells in a state of irreversible arrest by contributing to a persistent activation of a DNA damage response.<ref name="New York, N"/><ref>{{cite journal |last1=Passos |first1=João F |last2=Nelson |first2=Glyn |last3=Wang |first3=Chunfang |last4=Richter |first4=Torsten |last5=Simillion |first5=Cedric |last6=Proctor |first6=Carole J |last7=Miwa |first7=Satomi |last8=Olijslagers |first8=Sharon |last9=Hallinan |first9=Jennifer |last10=Wipat |first10=Anil |last11=Saretzki |first11=Gabriele |last12=Rudolph |first12=Karl Lenhard |last13=Kirkwood |first13=Tom B L |last14=von Zglinicki |first14=Thomas |title=Feedback between p21 and reactive oxygen production is necessary for cell senescence |journal=Molecular Systems Biology |date=January 2010 |volume=6 |issue=1 |pages=347 |doi=10.1038/msb.2010.5}}</ref> || |
|- | |- | ||
| − | | 2010 || Organization (research network) || || The {{w|European Network in Aging Studies}} is established.<ref name="sdeadf">{{cite web |title=Home |url=https://www.trentaging2019.com/ |website=TrentAging2019 |access-date=9 June 2021 |language=en}}</ref><ref>{{cite web |title=ENAS - home |url=http://www.agingstudies.eu/page/History#:~:text=The%20European%20Network%20in%20Aging,program%20Internationalization%20in%20the%20Humanities. |website=www.agingstudies.eu |access-date=9 June 2021}}</ref> || {{w|Europe}} | + | | 2010 || Organization (research network) || || The {{w|European Network in Aging Studies}} is established.<ref name="sdeadf">{{cite web |title=Home |url=https://www.trentaging2019.com/ |website=TrentAging2019 |access-date=9 June 2021 |language=en}}</ref><ref>{{cite web |title=ENAS - home |url=http://www.agingstudies.eu/page/History#:~:text=The%20European%20Network%20in%20Aging,program%20Internationalization%20in%20the%20Humanities. |website=www.agingstudies.eu |access-date=9 June 2021}}</ref> It is a research network that connects researchers with the purpose to facilitate sustainable international and multi-disciplinary collaboration among all researchers interested in the study of cultural aging.<ref>{{cite web |title=ENAS - home |url=https://www.agingstudies.eu/ |website=www.agingstudies.eu |access-date=23 December 2021}}</ref> || {{w|Europe}} |
|- | |- | ||
| − | | | + | | 2011 || Scientific development || Laboratory research || Study by Baker, Wijshake, and Tchkonia et al.<ref>{{cite journal |last1=Baker |first1=Darren J. |last2=Wijshake |first2=Tobias |last3=Tchkonia |first3=Tamar |last4=LeBrasseur |first4=Nathan K. |last5=Childs |first5=Bennett G. |last6=van de Sluis |first6=Bart |last7=Kirkland |first7=James L. |last8=van Deursen |first8=Jan M. |title=Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders |journal=Nature |date=November 2011 |volume=479 |issue=7372 |pages=232–236 |doi=10.1038/nature10600}}</ref> (confirmed later by Baker, Childs, and Durik et al. in 2016<ref>{{cite journal |last1=Baker |first1=Darren J. |last2=Childs |first2=Bennett G. |last3=Durik |first3=Matej |last4=Wijers |first4=Melinde E. |last5=Sieben |first5=Cynthia J. |last6=Zhong |first6=Jian |last7=A. Saltness |first7=Rachel |last8=Jeganathan |first8=Karthik B. |last9=Verzosa |first9=Grace Casaclang |last10=Pezeshki |first10=Abdulmohammad |last11=Khazaie |first11=Khashayarsha |last12=Miller |first12=Jordan D. |last13=van Deursen |first13=Jan M. |title=Naturally occurring p16Ink4a-positive cells shorten healthy lifespan |journal=Nature |date=February 2016 |volume=530 |issue=7589 |pages=184–189 |doi=10.1038/nature16932}}</ref>) reports that senescent cells accumulate with age and at sites of pathology, where they are shown to drive tissue deterioration.<ref name="New York, N"/> || {{w|United States}} ({{w|Mayo Clinic College of Medicine and Science}}, {{w|Rochester, Minnesota}}) |
|- | |- | ||
| − | | 2012 || Scientific development || Laboratory || Research by Nelson et al. shows that the bystander effect of senescent cells negatively affects non‐senescent cells via reactive oxygen species (ROS). The researchers also show that a paracrine effect of senescent cells can damage {{w|DNA}}.<ref name="Ogrodnik"/><ref>{{cite journal |last1=Nelson |first1=Glyn |last2=Wordsworth |first2=James |last3=Wang |first3=Chunfang |last4=Jurk |first4=Diana |last5=Lawless |first5=Conor |last6=Martin‐Ruiz |first6=Carmen |last7=von Zglinicki |first7=Thomas |title=A senescent cell bystander effect: senescence‐induced senescence |journal=Aging Cell |date=April 2012 |volume=11 |issue=2 |pages=345–349 |doi=10.1111/j.1474-9726.2012.00795.x}}</ref> || | + | | 2012 || Scientific development || Laboratory research || Researchers from the Institute of Molecular Oncology (IFOM) in {{w|Milan}} and Molecular Genetics of the National Research Council (IGM-CNR) in {{w|Pavia}} identify for the first time a class of non-coding RNAs, called “DNA Damage Response RNAs (DDRNAs)”, laying the foundation for the future advances in cellular aging.<ref>{{cite web |title=IFOM: research on cellular aging opens new therapeutic perspectives for various diseases |url=https://www.researchitaly.it/en/success-stories/ifom-research-on-cellular-aging-opens-new-therapeutic-perspectives-for-various-diseases/ |website=ResearchItaly - IFOM: research on cellular aging opens new therapeutic perspectives for various diseases |access-date=12 June 2021 |language=en}}</ref> || {{w|Italy}} |
| + | |- | ||
| + | | 2012 || Scientific development || Laboratory research || Research by Nelson et al. shows that the bystander effect of senescent cells negatively affects non‐senescent cells via reactive oxygen species (ROS). The researchers also show that a paracrine effect of senescent cells can damage {{w|DNA}}.<ref name="Ogrodnik"/><ref>{{cite journal |last1=Nelson |first1=Glyn |last2=Wordsworth |first2=James |last3=Wang |first3=Chunfang |last4=Jurk |first4=Diana |last5=Lawless |first5=Conor |last6=Martin‐Ruiz |first6=Carmen |last7=von Zglinicki |first7=Thomas |title=A senescent cell bystander effect: senescence‐induced senescence |journal=Aging Cell |date=April 2012 |volume=11 |issue=2 |pages=345–349 |doi=10.1111/j.1474-9726.2012.00795.x}}</ref> || {{w|United Kingdom}} ({{w|Newcastle University}}) | ||
|- | |- | ||
| 2013 (January) || Organization || || The North American Network in Aging Studies (NANAS) is established with the ultimate purpose to improve the health, care, and quality of life for people aging into old age.<ref name="sdeadf"/> || {{w|United States}} | | 2013 (January) || Organization || || The North American Network in Aging Studies (NANAS) is established with the ultimate purpose to improve the health, care, and quality of life for people aging into old age.<ref name="sdeadf"/> || {{w|United States}} | ||
|- | |- | ||
| − | | 2013 || Scientific development || Laboratory || A group of scientists define nine hallmarks of aging that are common between organisms with emphasis on mammals: | + | | 2013 || Scientific development || Laboratory research || A group of scientists define nine hallmarks of aging that are common between organisms with emphasis on mammals: |
* {{w|genomic instability}}, | * {{w|genomic instability}}, | ||
* [[w:telomere|telomere attrition]], | * [[w:telomere|telomere attrition]], | ||
| Line 342: | Line 321: | ||
* stem cell exhaustion, | * stem cell exhaustion, | ||
* altered intercellular communication.<ref name="pmid23746838">{{cite journal|year=2013|title=The hallmarks of aging|journal=Cell|volume=153|issue=6|pages=1194–217|doi=10.1016/j.cell.2013.05.039|pmc=3836174|pmid=23746838|author=Lopez-Otin, C|display-authors=etal}}</ref> | * altered intercellular communication.<ref name="pmid23746838">{{cite journal|year=2013|title=The hallmarks of aging|journal=Cell|volume=153|issue=6|pages=1194–217|doi=10.1016/j.cell.2013.05.039|pmc=3836174|pmid=23746838|author=Lopez-Otin, C|display-authors=etal}}</ref> | ||
| − | || | + | || |
|- | |- | ||
| − | | 2013 || | + | | 2013 || Scientific development || [[w:Category:Theories of biological aging|Theory]] || Silvertown suggests that the ways in which senescence is thought to evolve precludes any common mechanism of decline.<ref name="Shefferson"/> || |
|- | |- | ||
| − | | 2014 || Scientific development || Laboratory || Researchers report first evidence that [[w:Medication|pharmacological activation]] of SIRT1 extends lifespan in mice and improves their health.<ref name="Zainabadi" /><ref>{{cite journal|last1=Mitchell |first1=Sarah J |last2=Martin-Montalvo |first2=Alejandro |last3=Mercken |first3=Evi M |last4=Palacios |first4=Hector H |last5=Ward |first5=Theresa M |last6=Abulwerdi |first6=Gelareh |display-authors=3 |date=27 February 2014 |title=The SIRT1 Activator SRT1720 Extends Lifespan and Improves Health of Mice Fed a Standard Diet |url= |journal={{w|Cell Reports}} |volume=6 |issue=5 |pages=836–843 |doi=10.1016/j.celrep.2014.01.031 |pmc=4010117 |pmid=24582957}}</ref><ref>{{cite journal|last1=Mercken |first1=Evi M |last2=Mitchell |first2=Sarah J |last3=Martin-Montalvo |first3=Alejandro |last4=Minor |first4=Robin K |last5=Almeida |first5=Maria |last6=Gomes |first6=Ana P |display-authors=3 |date=16 June 2014 |title=SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass |url= |journal={{w|Aging Cell}} |volume=13 |issue=5 |pages=787–796 |doi=10.1111/acel.12220 |pmc=4172519 |pmid=24931715}}</ref> || | + | | 2013 || Scientific development || Laboratory research || Nussey et al. conduct literature survey, and find clear evidence of actuarial senescence (senescence in survival) in large mammalian species (i.e. ungulates, marine mammals and primates).<ref name="Shefferson"/> || |
| + | |- | ||
| + | | 2014 || Scientific development || Laboratory research || Researchers report first evidence that [[w:Medication|pharmacological activation]] of SIRT1 extends lifespan in mice and improves their health.<ref name="Zainabadi" /><ref>{{cite journal|last1=Mitchell |first1=Sarah J |last2=Martin-Montalvo |first2=Alejandro |last3=Mercken |first3=Evi M |last4=Palacios |first4=Hector H |last5=Ward |first5=Theresa M |last6=Abulwerdi |first6=Gelareh |display-authors=3 |date=27 February 2014 |title=The SIRT1 Activator SRT1720 Extends Lifespan and Improves Health of Mice Fed a Standard Diet |url= |journal={{w|Cell Reports}} |volume=6 |issue=5 |pages=836–843 |doi=10.1016/j.celrep.2014.01.031 |pmc=4010117 |pmid=24582957}}</ref><ref>{{cite journal|last1=Mercken |first1=Evi M |last2=Mitchell |first2=Sarah J |last3=Martin-Montalvo |first3=Alejandro |last4=Minor |first4=Robin K |last5=Almeida |first5=Maria |last6=Gomes |first6=Ana P |display-authors=3 |date=16 June 2014 |title=SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass |url= |journal={{w|Aging Cell}} |volume=13 |issue=5 |pages=787–796 |doi=10.1111/acel.12220 |pmc=4172519 |pmid=24931715}}</ref> || | ||
|- | |- | ||
| − | | | + | | 2014 || Scientific development || Laboratory research || Study by Correia-Melo, Hewitt, and Passos indicates that senescence is a multilayered process which involves dramatic time-dependent changes in gene expression, epigenetic and metabolic profile.<ref name="New York, N">{{cite book |title=Cellular senescence : methods and protocols |date=2018 |location=New York, NY |isbn=9781493989317}}</ref> || |
|- | |- | ||
| − | | | + | | 2015 || Scientific development || Laboratory research || Study by Madeo, Zimmermann, Maiuri and Kroemer determines that {{w|autophagy}} plays an important role during senescence through the elimination of old or unneeded materials.<ref>{{cite journal |last1=Madeo |first1=Frank |last2=Zimmermann |first2=Andreas |last3=Maiuri |first3=Maria Chiara |last4=Kroemer |first4=Guido |title=Essential role for autophagy in life span extension |journal=Journal of Clinical Investigation |date=2 January 2015 |volume=125 |issue=1 |pages=85–93 |doi=10.1172/JCI73946}}</ref> || |
|- | |- | ||
| − | | | + | | 2015 || Scientific development || Laboratory research || Study by Xu, Palmer, and Ding et al.<ref>{{cite journal |last1=Xu |first1=Ming |last2=Palmer |first2=Allyson K |last3=Ding |first3=Husheng |last4=Weivoda |first4=Megan M |last5=Pirtskhalava |first5=Tamar |last6=White |first6=Thomas A |last7=Sepe |first7=Anna |last8=Johnson |first8=Kurt O |last9=Stout |first9=Michael B |last10=Giorgadze |first10=Nino |last11=Jensen |first11=Michael D |last12=LeBrasseur |first12=Nathan K |last13=Tchkonia |first13=Tamar |last14=Kirkland |first14=James L |title=Targeting senescent cells enhances adipogenesis and metabolic function in old age |journal=eLife |date=19 December 2015 |volume=4 |pages=e12997 |doi=10.7554/eLife.12997}}</ref> (later confirmed by Baker, Childs, and Durik et al.<ref>{{cite journal |last1=Baker |first1=Darren J. |last2=Childs |first2=Bennett G. |last3=Durik |first3=Matej |last4=Wijers |first4=Melinde E. |last5=Sieben |first5=Cynthia J. |last6=Zhong |first6=Jian |last7=A. Saltness |first7=Rachel |last8=Jeganathan |first8=Karthik B. |last9=Verzosa |first9=Grace Casaclang |last10=Pezeshki |first10=Abdulmohammad |last11=Khazaie |first11=Khashayarsha |last12=Miller |first12=Jordan D. |last13=van Deursen |first13=Jan M. |title=Naturally occurring p16Ink4a-positive cells shorten healthy lifespan |journal=Nature |date=February 2016 |volume=530 |issue=7589 |pages=184–189 |doi=10.1038/nature16932}}</ref>) indicates that the accumulation of senescent cells in multiple tissues contributes causally to age-related tissue dysfunction.<ref name="New York, N"/> || {{w|United States}} (Robert and Arlene Kogod Center on Aging, {{w|Mayo Clinic}}, {{w|Rochester, Minnesota}}) |
|- | |- | ||
| − | | | + | | 2016 || Scientific development || Laboratory research || Scientists at {{w|Newcastle University}} demonstrate for the first time that {{w|mitochondria}} are major triggers of cell aging, after having found that when mitochondria are eliminated from aging cells, the latter become much more similar to younger cells. This discovery advances a step closer to developing therapies to counteract the aging of cells, by targeting mitochondria.<ref>{{cite journal |author=Clara Correia‐Melo, Francisco DM Marques, Rhys Anderson, Graeme Hewitt, Rachael Hewitt, John Cole, Bernadette M Carroll, Satomi Miwa, Jodie Birch, Alina Merz, Michael D Rushton, Michelle Charles, Diana Jurk, Stephen WG Tait, Rafal Czapiewski, Laura Greaves, Glyn Nelson, Mohammad Bohlooly‐Y, Sergio Rodriguez‐Cuenca, Antonio Vidal‐Puig, Derek Mann, Gabriele Saretzki, Giovanni Quarato, Douglas R Green, Peter D Adams, Thomas von Zglinicki, Viktor I Korolchuk, João F Passos |date= |title=Mitochondria are required for pro‐aging features of the senescent phenotype |url=http://emboj.embopress.org/content/35/7/724 |journal=The EMBO Journal |publisher= |volume= 35|issue= |pages= 724–742|doi=10.15252/embj.201592862 |pmc= |pmid= 26848154 |year=2016}}</ref><ref>{{cite web |title=Mitochondria shown to trigger cell aging: Batteries of the cells shown to be essential for aging |url=https://www.sciencedaily.com/releases/2016/02/160204084958.htm |website=ScienceDaily |access-date=7 June 2021 |language=en}}</ref><ref>{{cite web |last1=Writer |first1=GEN Staff |title=Mitochondria Trigger Cell Aging Response |url=https://www.genengnews.com/news/mitochondria-trigger-cell-aging-response/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=7 June 2021 |date=2016-02-04}}</ref> || {{w|United Kingdom}} ([[wikipedia:Newcastle University|Newcastle University]]) |
|- | |- | ||
| − | | | + | | 2016 || Scientific development || Laboratory research || Researchers find that the replenishment of nicotinamide adenine dinucleotide ([[w:Nicotinamide adenine dinucleotide|NAD<sup>+</sup>]]) in the organism of mice through precursor molecules improves the functioning of [[w:Mitochondrion|mitochondria]] and {{w|stem cell}}s, and also leads to an increase in their lifespan.<ref name="Zainabadi" /><ref>{{cite journal |last1=Zhang |first1=H. |last2=Ryu |first2=D. |last3=Wu |first3=Y. |last4=Gariani |first4=K. |last5=Wang |first5=X. |last6=Luan |first6=P. |last7=DAmico |first7=D. |last8=Ropelle |first8=E. R. |last9=Lutolf |first9=M. P. |last10=Aebersold |first10=R. |last11=Schoonjans |first11=K. |last12=Menzies |first12=K. J. |last13=Auwerx |first13=J. |title=NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice |journal=Science |date=17 June 2016 |volume=352 |issue=6292 |pages=1436–1443 |doi=10.1126/science.aaf2693}}</ref> One of these NAD<sup>+</sup> precursor molecules is nicotinamide mononucleotide [[w:Nicotinamide mononucleotide|NMN]].<ref>{{cite journal|last1=Yoshino |first1=Jun |last2=Mills |first2=Kathryn F. |last3=Yoon |first3=Myeong Jin |last4=Imai |first4=Shin-ichiro |date=15 October 2011 |title=Nicotinamide mononucleotide, a key NAD<sup>+</sup> intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice |journal={{w|Cell Metabolism}} |volume=14 |issue=4 |pages=528–536 |doi=10.1016/j.cmet.2011.08.014 |pmc=3204926 |pmid= 21982712}}</ref><ref>{{cite web|url=https://www.nmn.com/precursors/what-is-nmn |title=What is NMN? |work=NMN.com |date=5 May 2020}}</ref> || |
|- | |- | ||
| − | | 2018 (January) || Scientific development || Laboratory || Researchers at the {{w|University of Texas Health Science Center}} (UTHealth) in {{w|Houston}} report a connection between accelerated epigenetic aging and bipolar disorder. The results could explain why people suffering from bipolar disorder are more likely to die from age-related diseases.<ref>{{cite web|title=Bipolar Disorder Linked to Accelerated Epigenetic Aging|url=https://www.whatisepigenetics.com/bipolar-disorder-linked-accelerated-epigenetic-aging/|website=whatisepigenetics.com|accessdate=3 January 2018}}</ref> || {{w|United States}} | + | | 2016 || Scientific development || Laboratory research || Researchers demonstrate that a combination of longevity associated drugs can additively extend lifespan in mice.<ref name="Zainabadi" /><ref>{{cite journal |last1=Strong |first1=Randy |last2=Miller |first2=Richard A. |last3=Antebi |first3=Adam |last4=Astle |first4=Clinton M. |last5=Bogue |first5=Molly |last6=Denzel |first6=Martin S. |last7=Fernandez |first7=Elizabeth |last8=Flurkey |first8=Kevin |last9=Hamilton |first9=Karyn L. |last10=Lamming |first10=Dudley W. |last11=Javors |first11=Martin A. |last12=Magalhães |first12=João Pedro |last13=Martinez |first13=Paul Anthony |last14=McCord |first14=Joe M. |last15=Miller |first15=Benjamin F. |last16=Müller |first16=Michael |last17=Nelson |first17=James F. |last18=Ndukum |first18=Juliet |last19=Rainger |first19=G. Ed. |last20=Richardson |first20=Arlan |last21=Sabatini |first21=David M. |last22=Salmon |first22=Adam B. |last23=Simpkins |first23=James W. |last24=Steegenga |first24=Wilma T. |last25=Nadon |first25=Nancy L. |last26=Harrison |first26=David E. |title=Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α‐glucosidase inhibitor or a Nrf2‐inducer |journal=Aging Cell |date=October 2016 |volume=15 |issue=5 |pages=872–884 |doi=10.1111/acel.12496}}</ref> || |
| + | |- | ||
| + | | 2016 || Scientific development || Laboratory research || Study by Childs, Baker, and Wijshake et al. concludes that senescent intimal foam cells are deleterious at all stages of {{w|atherosclerosis}}.<ref name="New York, N"/><ref>{{cite journal |last1=Childs |first1=B. G. |last2=Baker |first2=D. J. |last3=Wijshake |first3=T. |last4=Conover |first4=C. A. |last5=Campisi |first5=J. |last6=van Deursen |first6=J. M. |title=Senescent intimal foam cells are deleterious at all stages of atherosclerosis |journal=Science |date=28 October 2016 |volume=354 |issue=6311 |pages=472–477 |doi=10.1126/science.aaf6659}}</ref> || | ||
| + | |- | ||
| + | | 2016 || Scientific development || Laboratory research || Correia-Melo, Marques, Anderson et al. determine that {{w|mitochondria}} are the powerhouses of cells and are essential for the development of cellular senescence.<ref name="New York, N"/><ref>{{cite journal |last1=Correia‐Melo |first1=Clara |last2=Marques |first2=Francisco DM |last3=Anderson |first3=Rhys |last4=Hewitt |first4=Graeme |last5=Hewitt |first5=Rachael |last6=Cole |first6=John |last7=Carroll |first7=Bernadette M |last8=Miwa |first8=Satomi |last9=Birch |first9=Jodie |last10=Merz |first10=Alina |last11=Rushton |first11=Michael D |last12=Charles |first12=Michelle |last13=Jurk |first13=Diana |last14=Tait |first14=Stephen WG |last15=Czapiewski |first15=Rafal |last16=Greaves |first16=Laura |last17=Nelson |first17=Glyn |last18=Bohlooly‐Y |first18=Mohammad |last19=Rodriguez‐Cuenca |first19=Sergio |last20=Vidal‐Puig |first20=Antonio |last21=Mann |first21=Derek |last22=Saretzki |first22=Gabriele |last23=Quarato |first23=Giovanni |last24=Green |first24=Douglas R |last25=Adams |first25=Peter D |last26=Zglinicki |first26=Thomas |last27=Korolchuk |first27=Viktor I |last28=Passos |first28=João F |title=Mitochondria are required for pro‐ageing features of the senescent phenotype |journal=The EMBO Journal |date=April 2016 |volume=35 |issue=7 |pages=724–742 |doi=10.15252/embj.201592862}}</ref> || | ||
| + | |- | ||
| + | | 2017 || Scientific development || Laboratory research || Researchers discover that a naturally occurring [[w:Polymorphism (biology)|polymorphism]] in human signaling pathways is in some cases associated with health and longevity. It is also detected that, the same as in mice, this association can depend on the gender (it can be observed for one gender but not for another). This indicates that by correctly influencing these pathways, it is theoretically possible to alter lifespan and healthspan in humans.<ref name="Zainabadi" /><ref>{{cite journal|last1=Ben-Avraham |first1=Danny |last2=Govindaraju |first2=Diddahally R. |last3=Budagov |first3=Temuri |last4=Fradin |first4=Delphine |last5=Durda |first5=Peter |last6=Liu |first6=Bing |last7=Ott |first7=Sandy |last8=Gutman |first8=Danielle |last9= Sharvit |first9=Lital |last10=Kaplan |first10=Robert |last11=Bougnères |first11=Pierre |last12=Reiner |first12=Alex |last13=Shuldiner |first13=Alan R. |last14=Cohen |first14=Pinchas |last15=Barzilai |first15=Nir |last16=Atzmon |first16=Gil |display-authors=5 |date=2 June 2017 |title=The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature |url= |journal={{w|Science Advances}} |volume=3 |issue=6 |pages= e1602025|doi=10.1126/sciadv.1602025 |pmc=5473676 |pmid=28630896}}</ref> || | ||
| + | |- | ||
| + | | 2017 || Scientific development || Laboratory research || Study by Jeon, Kim, and Laberge et al. reports that senescent cells accumulate in many vertebrate tissues with age and contribute to age-related pathologies, presumably through their secretion of factors contributing to the senescence-associated secretory phenotype,<ref>{{cite journal |last1=Jeon |first1=Ok Hee |last2=Kim |first2=Chaekyu |last3=Laberge |first3=Remi-Martin |last4=Demaria |first4=Marco |last5=Rathod |first5=Sona |last6=Vasserot |first6=Alain P |last7=Chung |first7=Jae Wook |last8=Kim |first8=Do Hun |last9=Poon |first9=Yan |last10=David |first10=Nathaniel |last11=Baker |first11=Darren J |last12=van Deursen |first12=Jan M |last13=Campisi |first13=Judith |last14=Elisseeff |first14=Jennifer H |title=Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment |journal=Nature Medicine |date=June 2017 |volume=23 |issue=6 |pages=775–781 |doi=10.1038/nm.4324}}</ref> the latter which defines the ability of senescent cells to express and secrete a variety of extracellular modulators that includes cytokines, chemokines, proteases, growth factors and bioactive lipids.<ref>{{cite journal |last1=Lopes-Paciencia |first1=Stéphane |last2=Saint-Germain |first2=Emmanuelle |last3=Rowell |first3=Marie-Camille |last4=Ruiz |first4=Ana Fernández |last5=Kalegari |first5=Paloma |last6=Ferbeyre |first6=Gerardo |title=The senescence-associated secretory phenotype and its regulation |journal=Cytokine |date=May 2019 |volume=117 |pages=15–22 |doi=10.1016/j.cyto.2019.01.013}}</ref> || | ||
| + | |- | ||
| + | | 2017 (December) || Scientific development || Laboratory research || Study shows that preventing {{w|wrinkle}}s could be as easy as expressing a protein called FKBP1b.<ref>{{cite web |last1=Matthews |first1=Stephen |title=Restricting calories can delay aging, says researcher |url=https://www.dailymail.co.uk/health/article-5199019/Restricting-calories-delay-aging-says-researcher.html |website=Mail Online |access-date=16 March 2021 |date=21 December 2017}}</ref> || | ||
| + | |- | ||
| + | | 2018 (January) || Scientific development || Laboratory research || Researchers at the {{w|University of Texas Health Science Center}} (UTHealth) in {{w|Houston}} report a connection between accelerated epigenetic aging and bipolar disorder. The results could explain why people suffering from bipolar disorder are more likely to die from age-related diseases.<ref>{{cite web|title=Bipolar Disorder Linked to Accelerated Epigenetic Aging|url=https://www.whatisepigenetics.com/bipolar-disorder-linked-accelerated-epigenetic-aging/|website=whatisepigenetics.com|accessdate=3 January 2018}}</ref> || {{w|United States}} | ||
|- | |- | ||
| 2018 || Policy || || The {{w|World Health Organization}} includes in the international classification of diseases {{w|ICD-11}} a special additional code signaling the relationship of a disease with age. As a result, after the final approval of the ICD-11 in May 2019, aging would begin to be officially recognized as a fundamental factor that increases the risk of diseases, the severity of their course and the difficulty of treatment.<ref>{{cite journal |title=Opening the door to treating ageing as a disease |journal=The Lancet Diabetes & Endocrinology |date=August 2018 |volume=6 |issue=8 |pages=587 |doi=10.1016/S2213-8587(18)30214-6}}</ref><ref>{{cite web|author=Biogerontology Research Foundation |url=https://www.eurekalert.org/pub_releases/2018-07/brf-who070218.php |title=World Health Organization adds extension code for 'aging-related' via ICD-11 |work={{w|American Association for the Advancement of Science#EurekAlert!}} |date=2 July 2018}}</ref><ref>{{cite web|author=Steve Hill |url=https://www.lifespan.io/news/a-step-closer-to-aging-being-classified-as-a-disease/ |title=Getting Aging Classified as a Disease – Daria Khaltourina |work={{w|Life Extension Advocacy Foundation}} |date=31 August 2018}}</ref><ref>{{cite web|url=https://www.fightaging.org/archives/2018/09/inching-towards-the-regulatory-classification-of-aging-as-a-disease/ |title=Inching Towards the Regulatory Classification of Aging as a Disease |website=Fight Aging! |date=3 September 2018}}</ref><ref>{{cite web|author=Oksana Andreiuk |url=https://medium.com/@oandreiuk/lets-talk-about-the-world-health-organisation-recognising-ageing-as-a-disease-risk-factor-4ca8ac89e59b |title=Let’s talk about the World Health Organisation recognising ageing as a disease risk factor, updating the ICD for the first time in 35 years. |website=[[w:Medium (website)|Medium]] |date=12 September 2018}}</ref> || | | 2018 || Policy || || The {{w|World Health Organization}} includes in the international classification of diseases {{w|ICD-11}} a special additional code signaling the relationship of a disease with age. As a result, after the final approval of the ICD-11 in May 2019, aging would begin to be officially recognized as a fundamental factor that increases the risk of diseases, the severity of their course and the difficulty of treatment.<ref>{{cite journal |title=Opening the door to treating ageing as a disease |journal=The Lancet Diabetes & Endocrinology |date=August 2018 |volume=6 |issue=8 |pages=587 |doi=10.1016/S2213-8587(18)30214-6}}</ref><ref>{{cite web|author=Biogerontology Research Foundation |url=https://www.eurekalert.org/pub_releases/2018-07/brf-who070218.php |title=World Health Organization adds extension code for 'aging-related' via ICD-11 |work={{w|American Association for the Advancement of Science#EurekAlert!}} |date=2 July 2018}}</ref><ref>{{cite web|author=Steve Hill |url=https://www.lifespan.io/news/a-step-closer-to-aging-being-classified-as-a-disease/ |title=Getting Aging Classified as a Disease – Daria Khaltourina |work={{w|Life Extension Advocacy Foundation}} |date=31 August 2018}}</ref><ref>{{cite web|url=https://www.fightaging.org/archives/2018/09/inching-towards-the-regulatory-classification-of-aging-as-a-disease/ |title=Inching Towards the Regulatory Classification of Aging as a Disease |website=Fight Aging! |date=3 September 2018}}</ref><ref>{{cite web|author=Oksana Andreiuk |url=https://medium.com/@oandreiuk/lets-talk-about-the-world-health-organisation-recognising-ageing-as-a-disease-risk-factor-4ca8ac89e59b |title=Let’s talk about the World Health Organisation recognising ageing as a disease risk factor, updating the ICD for the first time in 35 years. |website=[[w:Medium (website)|Medium]] |date=12 September 2018}}</ref> || | ||
|- | |- | ||
| − | | 2018 || Scientific development || Laboratory || Researchers identify {{w|naked mole-rat}}s as the first mammal to defy the {{w|Gompertz–Makeham law of mortality}}, and achieve negligible senescence. It would be speculated however that this may be simply a "time-stretching" effect primarily due to their very slow (and cold-blooded and hypoxic) metabolism.<ref>{{cite journal |last1=Ruby |first1=J Graham |last2=Smith |first2=Megan |last3=Buffenstein |first3=Rochelle |title=Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age |journal=eLife |volume=7 |doi=10.7554/eLife.31157 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5783610/ |issn=2050-084X}}</ref><ref>{{cite journal |last1=Beltrán-Sánchez |first1=Hiram |last2=Finch |first2=Caleb |title=Age is just a number |journal=eLife |volume=7 |doi=10.7554/eLife.34427 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5783609/ |issn=2050-084X}}</ref> || || [[File:Nacktmull.jpg|thumb|center|150px]] | + | | 2018 || Scientific development || Laboratory research || Researchers identify {{w|naked mole-rat}}s as the first mammal to defy the {{w|Gompertz–Makeham law of mortality}}, and achieve negligible senescence. It would be speculated however that this may be simply a "time-stretching" effect primarily due to their very slow (and cold-blooded and hypoxic) metabolism.<ref>{{cite journal |last1=Ruby |first1=J Graham |last2=Smith |first2=Megan |last3=Buffenstein |first3=Rochelle |title=Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age |journal=eLife |volume=7 |doi=10.7554/eLife.31157 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5783610/ |issn=2050-084X}}</ref><ref>{{cite journal |last1=Beltrán-Sánchez |first1=Hiram |last2=Finch |first2=Caleb |title=Age is just a number |journal=eLife |volume=7 |doi=10.7554/eLife.34427 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5783609/ |issn=2050-084X}}</ref> || || [[File:Nacktmull.jpg|thumb|center|150px]] |
|- | |- | ||
| − | | 2019 || Scientific development || Laboratory || Researchers show that many major features of cellular senescence, such as the pro‐inflammatory phenotype, are dependent on the stable cell cycle arrest.<ref>{{cite journal |last1=Lopes-Paciencia |first1=Stéphane |last2=Saint-Germain |first2=Emmanuelle |last3=Rowell |first3=Marie-Camille |last4=Ruiz |first4=Ana Fernández |last5=Kalegari |first5=Paloma |last6=Ferbeyre |first6=Gerardo |title=The senescence-associated secretory phenotype and its regulation |journal=Cytokine |date=May 2019 |volume=117 |pages=15–22 |doi=10.1016/j.cyto.2019.01.013}}</ref><ref name="Ogrodnik"/> || | + | | 2019 || Scientific development || Laboratory research || Researchers show that many major features of cellular senescence, such as the pro‐inflammatory phenotype, are dependent on the stable cell cycle arrest.<ref>{{cite journal |last1=Lopes-Paciencia |first1=Stéphane |last2=Saint-Germain |first2=Emmanuelle |last3=Rowell |first3=Marie-Camille |last4=Ruiz |first4=Ana Fernández |last5=Kalegari |first5=Paloma |last6=Ferbeyre |first6=Gerardo |title=The senescence-associated secretory phenotype and its regulation |journal=Cytokine |date=May 2019 |volume=117 |pages=15–22 |doi=10.1016/j.cyto.2019.01.013}}</ref><ref name="Ogrodnik"/> || |
|- | |- | ||
| − | | 2019 || Scientific development || Laboratory || Scientists manage to extend the average lifespan of mice by breeding them using embryonic stem cells with extra-long telomeres. The finding is significant as no genetic modification is conducted.<ref>{{cite web |title=Scientists extend mice lifespan 12% by tweaking telomeres |url=https://bigthink.com/surprising-science/telomere-aging?rebelltitem=1#rebelltitem1 |website=Big Think |access-date=13 June 2021 |language=en |date=2019-10-22}}</ref><ref>{{cite web |title=Scientists extend mice lifespan 12% by tweaking telomeres |url=https://amazingstories.com/2019/10/scientists-extend-mice-lifespan-12-by-tweaking-telomeres/ |website=Amazing Stories |access-date=13 June 2021 |language=en-us |date=2019-10-24}}</ref><ref>{{cite web |last1=Nield |first1=David |title=Scientists Dramatically Extend The Lifespans of Mice in a Genius New Telomere Study |url=https://www.sciencealert.com/researchers-have-made-long-lived-mice-with-extended-chromosomes-inside-all-of-their-cells |website=ScienceAlert |access-date=13 June 2021 |language=en-gb}}</ref> || | + | | 2019 || Scientific development || Laboratory research || Scientists manage to extend the average lifespan of mice by breeding them using embryonic stem cells with extra-long telomeres. The finding is significant as no genetic modification is conducted.<ref>{{cite web |title=Scientists extend mice lifespan 12% by tweaking telomeres |url=https://bigthink.com/surprising-science/telomere-aging?rebelltitem=1#rebelltitem1 |website=Big Think |access-date=13 June 2021 |language=en |date=2019-10-22}}</ref><ref>{{cite web |title=Scientists extend mice lifespan 12% by tweaking telomeres |url=https://amazingstories.com/2019/10/scientists-extend-mice-lifespan-12-by-tweaking-telomeres/ |website=Amazing Stories |access-date=13 June 2021 |language=en-us |date=2019-10-24}}</ref><ref>{{cite web |last1=Nield |first1=David |title=Scientists Dramatically Extend The Lifespans of Mice in a Genius New Telomere Study |url=https://www.sciencealert.com/researchers-have-made-long-lived-mice-with-extended-chromosomes-inside-all-of-their-cells |website=ScienceAlert |access-date=13 June 2021 |language=en-gb}}</ref> || |
|- | |- | ||
| − | | 2019 (December 9) || Scientific development || Laboratory || Researchers at the {{w|Pasteur Institute}} identify the CSB protein, whose absence or dysfunction causes early aging, among other afflictions, in patients with Cockayne syndrome.<ref>{{cite web |title=Identification of a key protein linked to aging |url=https://www.sciencedaily.com/releases/2019/12/191209112138.htm |website=ScienceDaily |access-date=27 May 2021 |language=en}}</ref> || {{w|France}} | + | | 2019 (December 9) || Scientific development || Laboratory research || Researchers at the {{w|Pasteur Institute}} identify the CSB protein, whose absence or dysfunction causes early aging, among other afflictions, in patients with Cockayne syndrome.<ref>{{cite web |title=Identification of a key protein linked to aging |url=https://www.sciencedaily.com/releases/2019/12/191209112138.htm |website=ScienceDaily |access-date=27 May 2021 |language=en}}</ref> || {{w|France}} |
|- | |- | ||
| − | | 2020 (July) || Scientific development || Laboratory || Scientists, using public [[w:List of biological databases|biological data]] on 1.75 million people with known lifespans overall, identify 10 [[w:Locus (genetics)|genomic loci]] which appear to intrinsically influence {{w|healthspan}}, lifespan, and {{w|longevity}} – of which half have not been reported previously at [[w:Genome-wide association study|genome-wide significance]] and most being associated with {{w|cardiovascular disease}} – and identify [[w:Human iron metabolism|haem metabolism]] as a promising candidate for further research within the field. Their study suggests that high levels of iron in the blood likely reduce, and genes involved in metabolising iron likely increase healthy years of life in humans.<ref>{{cite journal |last1=Timmers |first1=Paul R. H. J. |last2=Wilson |first2=James F. |last3=Joshi |first3=Peter K. |last4=Deelen |first4=Joris |title=Multivariate genomic scan implicates novel loci and haem metabolism in human aging |journal=Nature Communications |date=December 2020 |volume=11 |issue=1 |pages=3570 |doi=10.1038/s41467-020-17312-3}}</ref> || {{w|United Kingdom}} || | + | | 2020 (July) || Scientific development || Laboratory research || Scientists, using public [[w:List of biological databases|biological data]] on 1.75 million people with known lifespans overall, identify 10 [[w:Locus (genetics)|genomic loci]] which appear to intrinsically influence {{w|healthspan}}, lifespan, and {{w|longevity}} – of which half have not been reported previously at [[w:Genome-wide association study|genome-wide significance]] and most being associated with {{w|cardiovascular disease}} – and identify [[w:Human iron metabolism|haem metabolism]] as a promising candidate for further research within the field. Their study suggests that high levels of iron in the blood likely reduce, and genes involved in metabolising iron likely increase healthy years of life in humans.<ref>{{cite journal |last1=Timmers |first1=Paul R. H. J. |last2=Wilson |first2=James F. |last3=Joshi |first3=Peter K. |last4=Deelen |first4=Joris |title=Multivariate genomic scan implicates novel loci and haem metabolism in human aging |journal=Nature Communications |date=December 2020 |volume=11 |issue=1 |pages=3570 |doi=10.1038/s41467-020-17312-3}}</ref> || {{w|United Kingdom}} || |
|- | |- | ||
| − | | 2020 (December) || Scientific development || Laboratory || Researchers at {{w|University of California, San Francisco}} report on an experimental drug called ISRIB, which restores memory function months after traumatic brain injury (TBI) in mice.<ref>{{cite web |title=Drug Reverses Age-Related Mental Decline Within Days In Mice |url=https://www.ucsf.edu/news/2020/12/419201/drug-reverses-age-related-mental-decline-within-days |website=Drug Reverses Age-Related Mental Decline Within Days {{!}} UC San Francisco |access-date=8 July 2021 |language=en}}</ref> || {{w|United States}} | + | | 2020 (December) || Scientific development || Laboratory research || Researchers at {{w|University of California, San Francisco}} report on an experimental drug called ISRIB, which restores memory function months after traumatic brain injury (TBI) in mice.<ref>{{cite web |title=Drug Reverses Age-Related Mental Decline Within Days In Mice |url=https://www.ucsf.edu/news/2020/12/419201/drug-reverses-age-related-mental-decline-within-days |website=Drug Reverses Age-Related Mental Decline Within Days {{!}} UC San Francisco |access-date=8 July 2021 |language=en}}</ref> || {{w|United States}} |
|- | |- | ||
|} | |} | ||
| + | |||
| + | == Visual and numerical data == | ||
| + | |||
| + | === Mentions on Google Scholar === | ||
| + | |||
| + | The table below summarizes per-year mentions of senescence–related topics (entries without quotation marks) on Google Scholar as of May 13, 2021. | ||
| + | |||
| + | {| class="sortable wikitable" | ||
| + | ! Year | ||
| + | ! Senescence | ||
| + | ! Aging | ||
| + | ! Longevity | ||
| + | ! Life extension | ||
| + | |- | ||
| + | | 1980 || 2,840 || 24,300 || 7,200 || 48,900 | ||
| + | |- | ||
| + | | 1985 || 3,760 || 36,300 || 9,210 || 43,400 | ||
| + | |- | ||
| + | | 1990 || 5,840 || 69,200 || 13,400 || 101,000 | ||
| + | |- | ||
| + | | 1995 || 8,150 || 114,000 || 18,300 || 131,000 | ||
| + | |- | ||
| + | | 2000 || 12,200 || 237,000 || 33,600 || 269,000 | ||
| + | |- | ||
| + | | 2002 || 13,400 || 302,000 || 41,600 || 299,000 | ||
| + | |- | ||
| + | | 2004 || 16,600 || 403,000 || 51,300 || 296,000 | ||
| + | |- | ||
| + | | 2006 || 21,500 || 470,000 || 62,900 || 341,000 | ||
| + | |- | ||
| + | | 2008 || 26,800 || 481,000 || 74,500 || 364,000 | ||
| + | |- | ||
| + | | 2010 || 33,500 || 587,000 || 85,400 || 397,000 | ||
| + | |- | ||
| + | | 2012 || 43,500 || 712,000 || 109,000 || 409,000 | ||
| + | |- | ||
| + | | 2014 || 47,900 || 653,000 || 103,000 || 366,000 | ||
| + | |- | ||
| + | | 2016 || 47,200 || 466,000 || 88,800 || 298,000 | ||
| + | |- | ||
| + | | 2017 || 45,300 || 441,000 || 81,500 || 254,000 | ||
| + | |- | ||
| + | | 2018 || 43,800 || 297,000 || 74,600 || 194,000 | ||
| + | |- | ||
| + | | 2019 || 39,500 || 201,000 || 59,400 || 148,000 | ||
| + | |- | ||
| + | | 2020 || 34,800 || 130,000 || 44,200 || 103,000 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | [[File:Senescence research.png|thumb|center|700px]] | ||
| + | |||
| + | === Google Trends === | ||
| + | |||
| + | The comparative chart below shows {{w|Google Trends}} data for Senescence (Topic), Cellular senescence (Topic) and Negligible senescence (Topic), from January 2004 to April 021, when the screenshot was taken. Interest is also ranked by country and displayed on world map.<ref>{{cite web |title=Senescence research |url=https://trends.google.com/trends/explore?date=all&q=%2Fm%2F012pdz,%2Fg%2F11c20bzs19,%2Fm%2F064lhv0 |website=Google Trends |access-date=16 April 2021}}</ref> | ||
| + | |||
| + | [[File:Senescence research gt.png|thumb|center|600px]] | ||
| + | |||
| + | === Google Ngram Viewer === | ||
| + | |||
| + | The chart below shows {{w|Google Ngram Viewer}} data for Senescence research, from 1950 to 2019.<ref>{{cite web |title=Senescence research |url=https://books.google.com/ngrams/graph?content=senescence+research&year_start=1950&year_end=2019&corpus=26&smoothing=3&direct_url=t1%3B%2Csenescence%20research%3B%2Cc0#t1%3B%2Csenescence%20research%3B%2Cc0 |website=books.google.com |access-date=16 April 2021 |language=en}}</ref> | ||
| + | |||
| + | [[File:Senescence research ngram.png|thumb|center|700px]] | ||
| + | |||
| + | === Wikipedia Views === | ||
| + | |||
| + | The chart below shows pageviews of the English Wikipedia article {{w|Senescence}}, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to March 2021.<ref>{{cite web |title=Senescence |url=https://wikipediaviews.org/displayviewsformultiplemonths.php?page=Senescence&allmonths=allmonths&language=en&drilldown=all |website=wikipediaviews.org |access-date=16 April 2021}}</ref> | ||
| + | |||
| + | [[File:Senescence wv.png|thumb|center|450px]] | ||
==Meta information on the timeline== | ==Meta information on the timeline== | ||
| Line 386: | Line 448: | ||
===What the timeline is still missing=== | ===What the timeline is still missing=== | ||
| + | ** Suggestions for expansion (from Vipul); don't limit yourself just to these: | ||
| + | *** Probably we should add more column(s) to classify by the theory or approach that the row is about. These columns may be blank for some rows.✔/✘ | ||
| + | *** There should be more context on individual rows, including possibly some context that explains the significance and current standing.✔/✘ The idea is that readers reading it should have a sense of the ups and downs of specific theories in the marketplace of ideas.✔/✘ | ||
| + | *** Added 2021-08-28: Big picture would also benefit from some summary around the different theories. | ||
| + | *** Maybe some charts of things based on Google Scholar✔, research budgets✘, etc. | ||
| − | + | * [https://bigthink.com/health/aging-research/#Echobox=1641103271] | |
| − | * [https:// | + | * [https://bigthink.com/health/aging-research/#Echobox=1649219461] |
| − | * [ | ||
| − | |||
===Timeline update strategy=== | ===Timeline update strategy=== | ||
Latest revision as of 17:33, 9 April 2024
This page is a timeline of senescence research, including major theories, breakthroughs and organizations. Senescence here is defined as "the process of physiological or biological decay leading to increasing mortality rates and/or decreasing fertility rates with age". This should be distinguished from "aging", which can be viewed simply as the march of time, with no physiological decay implied.
Contents
Sample questions
The following are some interesting questions that can be answered by reading this timeline:
- What are some of the numerous theories of aging having been proposed throughout history?
- Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Theory".
- You will mostly see the description of a selected number among hundreds of theories of aging, including the most relevant.
- What are some significant studies on senescence conducted in research labs?
- Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Laboratory research".
- You will see some notable lab discoveries related to the field of senescence.
- What are some other types of research mentioned in this timeline?
- Sort the full timeline by "Research type (when applicable)".
- Other types of research mentioned are: Clinical study, Concept development, and Data science.
- What are some organizations established with the purpose to promote the scientific development in the field of senescence?
- Sort the full timeline by "Event type" and look for the group of rows with value "Organization".
- You will see some non-profit organizations, other private, as well as some important governmental organizations focused on both senescence and aging.
- What are some notable publications specialized in the field of senescence?
- Sort the full timeline by "Event type" and look for the group of rows with value "Literature".
- You will see some publications by Leonard Hayflick, Roy Halford and Aubrey de Grey.
- For specialized jornals, sort the full timeline by "Event type" and look for the group of rows with value "Literature (journal)"
- What are some notable concepts having been introduced in the field of senescence?
- Sort the full timeline by "Research type (when applicable)" and look for the group of rows with value "Concept introduction".
- You will see descriptions around concepts like gerontology, senescence, and programmed cell-death.
- Other events are described under the following types: "Conference", "Policy", and "Recognition".
Big picture
| Year/period | Event |
|---|---|
| Ancient Greece | Early speculations on aging are focussed on the bodily humoral imbalance and on the gradual loss of inner heat.[1] |
| Middle Age/Renaissance | Rejuvenating or stopping the aging process is a major concern in this period.[1] |
| Renaissance–18th century | Some themes around which aging and senescence research revolve are the idea that senescence is itself an illness, the image of the aged body as a lamp in which life-fuel has run out, the character alterations of elders, and the attempt to prolong life through specific diet or by substituting damaged body parts.[1] |
| 19th century | The modern evolutionary theory of senescence is rooted in the late century.[2] Starting from the so-called "fin-de-siècle period, scientific optimism flourishes, and life-extensionism represents the most radical form of the trend.[3] Life expectancy starts to rise in the Western world.[4] |
| 20th century | Since the 1930s, it is understood that restricting calories can extend lifespan in laboratory animals.[5] After the Second World War, modern knowledge accumulates. The 1950s see the first attempts to distinguish, measure and compare the functional and chronological ages of individuals, of which biological considerations were an important component.[6][7] The beginning of the term “senescence” in the context of mammalian cell cultures begins in the 1960s with the work of Hayflick and Moorhead.[8] Also by this same decade, biologists start becoming aware of mortality leveling-off.[9] Around the start of the 1970s, senescence research starts broadening its evolutionary scope.[2] During the mid-1970s, there is considerable interest in the idea that cells might become destabilized and thereby precipitates onto a pathway of progressive deterioration.[2] In the 1980s, the field of life-span psychology is born.[10] In the 1990s, Caleb Finch gives serious consideration to organisms that exhibit negligible senescence and experience no, or only very small, increases in mortality rate with age.[2] |
| 21st century | Modern anti-aging organizations merge and their proliferation multiplies toward the 2000s.[3] In the 21st century, research activity increases, as well as interdisciplinary collaboration of senescence research with other fields, such as data science. In the second half of 2010s official discussions about the possibility of recognizing aging as a disease emerge.[11][12][13][14][15] |
Full timeline
| Year/period | Type of Event | Research type (when applicable) | Event | Location | |
|---|---|---|---|---|---|
| c. 99 BC – c. 55 BC | Scientific development | Theory | Roman poet and philosopher Lucretius argues that aging and death are beneficial because they make room for the next generation. This view will persist among biologists well into the 20th century.[16] | ||
| 5th century | Scientific development | Theory | Early formulations, described by Hippocrates' system of four humours, theorize old age as a consequence of the gradual consumption of the innate heat with the inevitable loss of body moisture.[1] | Greece | |
| 1825 | Scientific development | Theory | British mathematician Benjamin Gompertz proposes an exponential increase in death rates with age, giving birth to what later will be called The Gompertz-Makeham law.[17][18] The simplest form of the law is: p = a + bx. According to the law, the probability of death p is defined as the sum of age-independent component a and the component depending of age bx which with age increases exponentially. If we place organisms in an absolutely protected environment and in this way make the first component negligible, the probability of death will be completely defined by the second component which actually describes the probability to die from aging. | United Kingdom | |
| 1882 | Scientific development | Theory | German evolutionary biologist August Weismann introduces the wear and tear theory of aging, which sustains that cells and tissues have vital parts that wear out resulting in aging. "Like components of an aging car, parts of the body eventually wear out from repeated use, killing them and then the body."[5] Aging would result from an accumulation of damage to cells, tissues, and organs in the body caused by dietary toxins and environmental agents, leading to the weakening and eventual death of the cells, tissues, and organs.[19] | Germany | |
| 1891 | Scientific development | Theory | German evolutionary biologist August Weismann proposes the first formal programmed aging theory as an evolutionary explanation of aging driven by group selection. His argument is that aging evolved to the advantage of the species (e.g., by replacing worn out individuals with younger ones), not the individual.[20][21] | Germany | |
| 1903 | Scientific development | Concept introduction | Russian zoologist Ilya Mechnikov coins the term "gerontology".[22][23][24] | ||
| 1904 | Scientific development | Theory | Metchnikoff introduces the Autointoxication theory of aging, which is considered important at the whole animal level.[25] This is one of the first toxic theories as Metchnikoffs proposes the idea of autointoxication by products of bacterial origin, primarily from bacterial processes in the large intestine. Metchnikoff believes that even simple changes of acidity by the consumption of yogurt could inhibit the production of bacterial toxins.[26] | ||
| 1908 | Scientific development | Theory | German physiologist Max Rubner describes his rate-of-living theory, which proposes that a slow metabolism increases an animal's longevity. It states that fast basal metabolic rate corresponds to short maximum life span.[27][28] Some studies conclude that the rate‐of‐living theory does not hold true for terrestrial vertebrates, and suggest that life expectancy is driven by selection arising from extrinsic mortality factors.[29] Support for this theory is bolstered by studies linking a lower basal metabolic rate (evident with a lowered heartbeat) to increased life expectancy.[30][31] However, in a 2007 analysis it would be shown that, when modern statistical methods for correcting for the effects of body size and phylogeny are employed, metabolic rate does not correlate with longevity in mammals or birds.[32] | Germany | |
| 1922 | Scientific development | Theory | Ukrainian pathophysiologist Aleksandr Bogomolets proposes that the deterioration of the connective tissues cause aging.[33] | Ukraine (Soviet Union) | |
| 1928 | Scientific development | Theory | American biologist Raymond Pearl describes the rate of living hypothesis as an expansion of the earlier theory by Max Rubner. It states that organisms with a high metabolic rate have shorter lives.[34] Further studies would demonstrate that rats kept on restricted diets and in cold environments live longer and that these conditions have the appearance and behavior of younger animals.[35] | United States | |
| 1930 | Literature (journal) | The first world's journal about aging and longevity is established in Japan.It's titled Acta Gerontologica Japonica (Yokufuen Chosa Kenkyu Kiyo).[36] | Japan | ||
| 1934 | Scientific development | Laboratory research | Mary Crowell and Clive McCay at Cornell University discover that calorie restriction can extend lifespan twofold in rats.[37] | United States | |
| 1938 | Organization | The German Society for Aging Research (German: Deutsche Gesellschaft für Altersforschung) is founded in Leipzig as the first specialized society dedicated to the study of aging. The founder, Max Bürger, also establishes the specialized journal Zeitschrift für Altersforschung , which becomes the third such journal in the world after the previously mentioned Japanese and Romanian journals.[38] | Germany | ||
| 1938 | Conference | The world's first scientific conference on aging and longevity is held in Kiev.[39][40] | Ukraine (USSR at the time) | ||
| 1939 | Organization (non-profit) | The British Society for Research on Aging is founded by Russian-British gerontologist Vladimir Korenchevsky.[41] It promotes research to understand the causes and effects of the aging process.[42] | United Kingdom | ||
| 1940 | Scientific development | Theory | Nagorny introduces a theory of aging based on age changes, which is described as the stabilization and inactivation (insolubilization) of intercellular structural proteins. Nagorny postulates that aging is linked to the accumulation of intercellular inactivated "metaplasmic" proteins due to the inability of enzymes to destroy them.[26] | ||
| 1942 | Scientific development | Theory | Johan Björkstein proposes the cross‐linkage theory of aging. According to it, aging results from the accumulation of intra‐ and intermolecular covalent bonds between molecules, termed “cross‐links.”[43][5] Recent studies show that cross-linking reactions are involved in the age related changes in the studied proteins.[44] | ||
| 1945 | Scientific development | Theory | The idea that free radicals are toxic agents is first proposed by Argentinian biologist Rebeca Gerschman and colleagues.[45] | ||
| 1945 | Organization | The Gerontological Society of America is founded "to promote the scientific study of aging, to encourage exchanges among researchers and practitioners from the various disciplines related to gerontology, and to foster the use of gerontological research in forming public policy".[46] | United States | ||
| 1945–1949 | Scientific development | Laboratory research | The advent of molecular biology changes the theoretical perception of aging dramatically, as the precise molecular structure of proteins and genetic material becomes known.[3] | United States | |
| 1946 | Literature (journal) | The Journal of Gerontology is founded.[47] | United States | ||
| 1947 | Scientific development | Theory | Aleksandr Bogomolets links aging with the functional deterioration of connective tissues.[26] | ||
| 1950 | Organization | The International Association of Gerontology is formed.[48] | |||
| 1952 | Scientific development | Theory | British biologist Peter Medawar formulates the first modern theory of mammal aging, known as Mutation accumulation theory, whereby the mechanism of action involves random, detrimental germline mutations of a kind that happen to show their effect only late in life.[16] | ||
| 1954 | Scientific development | Theory | Soviet scientist Vladimir Dilman formulates the elevation hypothesis of aging, a hypothesis known only in the USSR at first. Later, in 1968, it would take the form and became known as the neuroendocrine theory of aging.[49][50][51] | Russia (USSR) | |
| 1954 | Scientific development | Theory | The Free-radical theory of aging (first introduced by Dr. Rebeca Gerschman) is developed by Dr. Denham Harman, this theory proposes that superoxide and other free radicals cause damage to the macromolecular components of the cell, giving rise to accumulated damage causing cells, and eventually organs, to stop functioning.[5] | ||
| 1954 | Scientific development | Theory | Research by Nikitin introduces a theory of aging based on age changes, and described as the accumulation of protein-DNA cross-links and progressive stabilization of chromatin complexes. This theory is further elaborated by von Hahn.[26] | ||
| 1956 | Scientific development | Theory | American chemist Denham Harman presents the free radical theory of aging, which states that organisms age over time due to the accumulation of damage from free radicals in the body.[34] Harman is known as the "father of the free radical theory of aging".[52][53] Today, an increasing number of studies contradict this theory.[54] | United States | |
| 1956 | Scientific development | Theory | Benefitted by the cosolidation of knowledge of the toxic intermediate products of normal metabolism, Vladimir Korenchevsky introduces a general toxic theory of aging.[26] | ||
| 1956 | Scientific development | Laboratory research | Puck and Markus solve the problem of identifying all forms of cell death, by developing an assay based on the ability of a single cell to grow into a colony. This "clonogenic assay" would form the basis of in vitro cellular response studies in tumors and also some normal tissues.[55] | ||
| 1957 | Scientific development | Theory | American evolutionary biologist George C. Williams proposes the today called Antagonistic pleiotropy hypothesis (AP) for the evolution of aging. It occurs when one gene controls for more than one phenotypic trait where at least one of these is beneficial to the organism's fitness and at least one is detrimental, thus accumulating damage.[16][56] This theory extends the argument by Peter Medawar.[2] | United States | |
| 1957 | Scientific development | Theory | M. F. Sinex publishes article titled Aging and the lability of irreplacable molecules, establishing a theory based on quantitative changes of proteins, and describing as the loss of irreplaceable molecules or enzymes.[26][57] | ||
| 1958 | Scientific development | Theory | American physicists Gioacchino Failla and Leo Szilard propose the somatic mutation theory, which suggests that aging is caused by random DNA damage in somatic cells and that the extent of damage is enhanced by radiation.[3] | United States | |
| 1958 | Scientific development | Clinical study | The Baltimore Longitudinal Study of Aging begins in the United States as a clinical research program on human aging. As of 2020, it is the longest-running study of aging in that country. It consists in volunteers of different ages in healthy conditions being follow-up visited for years. Participants are tested and evaluated for many physical elements as well as for brain function, mood, personality, and social aspects of life. This program would contribute more than any other research project to the understanding of aging.[58] | United States | |
| 1959 | Scientific development | Theory | Sacher introduces a size-lifespan correlation theory, positively correlating body weight with the longevity in mammals.[26] | ||
| 1959 | Scientific development | Theory | Roy Walford proposes the Immunological theory of aging,[59] which asserts that the process of human aging is largely controlled by the immune system, which weakens as an organism ages. This makes the organism unable to fight infections and less able to destroy old and neoplastic cells. This leads to aging and will eventually lead to death.[60] | United States | |
| 1961 | Scientific development | Concept introduction | The beginning of the term “senescence” in the context of mammalian cell cultures is considered to be born out of the discovery by American anatomist Leonard Hayflick and his colleague Paul Moorhead, who describe that primary cells have a finite lifespan when cultured in vitro, contrasting cancer cells that divide without limits.[8] Hayflick demonstrates that a population of normal human fetal cells in a cell culture will divide between 40 and 60 times before entering a senescence phase. This process will be known later as the Hayflick limit.[61][62][63] | United States | |
| 1961 | Scientific development | Theory | Vladimir Korenchevsky introduces the Endocrine theory of aging, which is considered a major theory at the organ level.[25] | ||
| 1962 | Scientific development | Theory | Romanian scientists Constantin Ion Parhon and Simion Oeriu introduce a theory of aging based on age changes, and described as the progressive demethylation of proteins.[26] | Romania | |
| 1963 | Scientific development | Theory | Leslie Orgel proposes the Error Catastrophe Theory of Aging, which is based on changes in protein biosynthesis (translation).[26] This theory states that aging is the result of the accumulation of errors in cellular molecules that are essential for cellular function and reproduction that eventually reaches a catastrophic level that is incompatible with cellular survival.[64][65] | ||
| 1963 | Scientific development | Laboratory research | South African biologist Sydney Brenner suggests the ability to easily and cheaply grow large quantities of worms in the lab as being very helpful for aging research, especially when identifying long-lived mutants caenorhabditis elegans, which have a relatively short lifespan (average approximately 17 days at 20 °C), and the lifespan is largely invariant.[66] | ||
| 1964 | Scientific development | Concept introduction | The concept of programmed cell death is introduced by American cellular biologist Richard A. Lockshin and Carroll Williams.[67] PCD consists in any active cellular process that culminates in cell death.[68] | United States | |
| 1964 | Scientific development | Theory | Romanian biochemist Simion Oeriu introduces a theory of aging based on age changes, described as accumulation of -S-S, inter- and intra- molecular bonds.[26] | Romania | |
| 1964 | Literature | British scientist Alex Comfort publishes Ageing – the Biology of Senescence, a classic book in the field. The book summarizes the state of the evolutionary study of senescence as lacking the scientific rigor seen in other disciplines. Comfort criticizes the field for yielding a number of evolutionary theories but failing to generate sufficient data to test those theories.[2] | |||
| 1965 | Scientific development | Theory | Leonard Hayflick describes cell senescence as the process that limits the number of cell divisions normal human cells can undergo in culture. This work formulates the The Cellular Senescence Theory of aging.[69][70] | ||
| 1965–1969 | Scientific development | Laboratory research | The strong effect of age on DNA methylation levels is discovered,[71] thus rendering it an accurate biological clock in humans and chimpanzees.[72] | ||
| 1966 | Scientific development | Theory | William Hamilton lays out the mathematics drawing on the ideas of George C. Williams and Peter Medawar, leading to the outcome that "senescence is an inevitable outcome of evolution".[2] | ||
| 1967 | Scientific development | Theory | C. Alexander sets the grounds of the DNA damage theory of aging by suggesting that DNA damage, as distinct from mutation, is the primary cause of aging.[73] This theory becomes stronger through further experimental support during the following decades.[74][75] | ||
| 1969 | Scientific development | Theory | American physician Roy Walford introduces the autoimmune theory of aging, which asserts that with age, the immune system tends to lose efficiency and experiences widespread dysfunction, evidenced by autoimmunity (immune reactions against one's own body proteins) and a decreased ability to respond to infection and other immune challenges.[76] It is well documented that the effectiveness of the immune system peaks at puberty and gradually declines thereafter with advance in age.[77] | United States | |
| 1970 | Scientific development | Theory | Hungarian-Canadian endocrinologist Hans Selye introduces the Stress damage theory, after observing a series of physiological changes in the rats after they were exposed to stressful events. Considered an important theory of aging at the whole animal level, this theory identifies three stages: alarm, resistance, and exhaustion.[25][78] | Canada | |
| 1970 | Organization | The American Aging Association is founded by Denham Harman. It is a non-profit, tax-exempt biogerontology organization of scientists and laypeople dedicated to biomedical aging studies and geroscience, with the goal of slowing the aging process to extend the healthy human lifespan while preserving and restoring functions typically lost to age-related degeneration.[79] | United States | ||
| 1972 | Scientific development | Concept development | Kerr, Wyllie, and Currie first coin the term “apoptosis” to differentiate naturally occurring developmental cell death from the necrosis that results from acute tissue injury. They also note that apoptosis is responsible for maintaining tissue homeostasis by mediating the equilibrium between cell proliferation and cell death in a particular tissue.[55] | ||
| 1974 | Organization | The National Institute on Aging (NIA) is formed as a division of the United States National Institutes of Health (NIH), with the purpose of conducting research on aging process and age-related diseases and disseminating information on health and research advances, among other aims.[80][81] | United States (National Institute on Aging) | ||
| 1974 | Scientific development | Theory | Study by Arthur B. Robinson introduces a theory of aging based on age changes, and described as the progressive deamination of glutaminyl and asparaginyl residues in proteins.[82][26] | United States | |
| 1975 | Scientific development | Theory | Sacher introduces a brain size–lifespan correlation theory, suggesting that larger brains make evolutionary selection of longer lifespan necessary.[26] | ||
| 1975 | Scientific development | Laboratory research | Australian-American molecular biologist Elizabeth Blackburn discovers the unusual nature of telomeres, with their simple repeated DNA sequences composing chromosome ends.[83][84] Some years later, Blackburn, Carol Greider and Jack Szostak discover how chromosomes are protected by telomeres and the enzyme telomerase, for which they receive the 2009 Nobel Prize in Physiology or Medicine.[85] Further experiments establish the role of telomere shortening in cellular aging and telomerase reactivation in cell immortalization.[86] | United States | |
| 1976 | Scientific development | Theory | Cutler introduces a theory of aging based on age changes, and describes it as the cross-links between DNA molecules.[26] | ||
| 1977 | Scientific development | Theory | English biologist Thomas Kirkwood proposes the third mainstream theory of aging, the disposable soma, which states that organisms only have a limited amount of energy that has to be divided between reproductive activities and the maintenance of the non-reproductive aspects of the organism.[87] Considered to be the most influential line of reasoning in gerontology, this theory states that aging is caused by accumulation of random damage, which is counteracted by repair. Repair is costly and the organism allocates exactly the needed amount of energetic resources. DST would be applied to explain why women live longer than men. Women are less disposable than men, so they need a better repair and thus live longer.[88] This theory would be further developed in substantial detail (Flatt et al. 2013; Kirkwood 1981, 1985, 2002, 2005; Kirkwood & Holliday 1979; Kirkwood & Rose 1991).[2] | ||
| 1977 | Scientific development | Theory | Research started by Kirkwood establishes an aging theory on lifespan correlations with changes at the molecular level. This theory suggests that immortal germ cells may have more comprehensive repairs and may have higher accuracy of synthesis than somatic cells.[26] | ||
| 1977 | Scientific development | Theory | Holliday and colleagues introduce the commitment theory of cellular aging, which stipulates the existence of a biological clock (pace-maker).[26] | ||
| 1977 | Scientific development | Laboratory research | Klass publishes that nematode C. elegans is a good system for aging studies as he establishes a method to consistently measure lifespan, concluding that this could lead to future detailed analysis combining genetics and biochemistry. Klass also finds that altering either temperature or the amount of food results in a change in lifespan. In addition, only small effects on lifespan are observed based on parental age or parental lifespan.[66] | ||
| 1977 | Scientific development | Theory | British biologist Thomas Kirkwood first proposes the disposable soma theory of aging in a Nature review article. This theory is inspired by Leslie Orgel's Error Catastrophe Theory of Aging.[89] | ||
| 1977 | Literature | American biologists Caleb Finch and Leonard Hayflick publish the Handbook of the Biology of Aging.[9] | United States | ||
| 1978 | Scientific development | Theory | Todd introduces a size-lifespan correlation theory, positively correlating size, height and longevity among tree species. He suggests a protective role of large sizes from disease, predators, etc.[26] | ||
| 1978 | Scientific development | Theory | Soviet scientist A. N. Lobachev proposes the mitochondrial non free radical theory of aging, which suggests that the main reason of accumulation of damages in mt DNA is the fact that at certain moment of cell life, the development of mitochondria begin to conflict with the development of nucleus. This theory concludes that mitochondria appears to be the «biologic clock» of the cell and programm the duration of its life.[90][91] | Russia | |
| 1978 | Scientific development | Laboratory research | For research on aging, early studies in nematode C. elegans focus on the feasibility of measuring lifespan and the use of 5-Fluoro-2′-deoxyuridine (FUDR) to maintain synchronous cultures of aged animals.[66] | ||
| 1979 | Scientific development | Theory | Vaupel et al. produce one of the earliest quantitative theories of lifelong heterogeneity. Under their model, the probability of an individual dying is described by a Gompertz equation, a sigmoid function which describes growth as being slowest at the start and end of a given time period.[2] | ||
| 1979 | Scientific development | Theory | Research by D. Gershon at Technion introduces a theory of aging based on age changes, and described as age-altered enzymes theory (age-related accumulation of conformational changes in protein leading to the inactivation of enzymes).[26] | Israel | |
| 1979 | Scientific development | Theory | Kirkwood and Holliday introduce a theory correlating lifespan with changes at the molecular level. Their hypothesis suggests higher fidelity of syntheses of macromolecules in longer-lived species and cellular clones.[26] | ||
| 1980 | Scientific development | Theory | M. S. Kanungo introduces a theory of aging based on age changes, describing it as the decrease of phosphorylation and acetylation of chromatin proteins as the cause of defects and decline in transcription.[26][92] | ||
| 1980 | Scientific development | Theory | Strehler introduces an aging theory classification that underlines the interrelation between different mechanisms of aging in an attempt to unite all these types into two "supergroups": genetically programmed aging, and aging secondary to genetic qualities (entropic increase). Strehler classification groups theories of aging into evolutionary (selection for limited lifespan), post-reproductive failure, failure of coordinating systems, pleiotropic side effects of advantageous qualities (clonal aging, autoimmunity), informational failure (changes in proteins and DNA), structural damage or loss and accumulation of dysfunctional materials (age-pigments, inactive proteins, lytic enzymes, etc.)[26] | ||
| 1981 | Scientific development | Lee Eberhardt stresses the need of including senescence when assessing population dynamics, as senescence is expected to have strong implications for conservation and management of mammalian populations. In particular, the occurrence of actuarial and reproductive senescence is expected to lead to a decrease in individual fitness and population growth rates.[2] | |||
| 1982 | Scientific development | Theory | Pashko and Schwartz propose an aging theory on lifespan correlations at the cellular level, suggesting a correlation between lifespan and species-specific activity of detoxification enzymes (longer-lived animals have higher efficiency of detoxification and are more resistant to environmental toxins).[26] | ||
| 1986 | Organization (non-profit) | The Alliance for Aging Research is founded as a non-profit organization, with the purpose to promote medical and behavioral research into the aging process.[93] | United States | ||
| 1986 | Scientific development | Theory | The Reliability theory of aging and longevity is proposed by Leonid Gavrilov and Natalia Gavrilova.[94][95][96][97] | ||
| 1987 | Scientific development | Laboratory research | B.M. Stanulis-Praeger determines cell death to be a primary consequence of senescence.[98] | United States (Tufts University, Boston) | |
| 1988 | Scientific development | Laboratory research | Genetic work by Tom Johnson et al. on mutant C. elegans mapps all of them to a single genetic locus, named age-1. This is the first breakthrough in aging research for studies based on C. elegans as this study reveals that it is possible to identify mutants that altered lifespan and more importantly, individual genes can modulate lifespan.[66] | ||
| 1988 | Scientific development | Theory | In a pioneering attempt to find quantitative evidence for senescence in wild populations of animals, Nesse concludes that it does in fact occur.[2] | ||
| 1989 | Scientific development | Theory | The network theory of aging is introduced. It supports the idea that multiple connected processes contribute to the biology of aging.[99] | ||
| 1990 | Scientific development | Theory | Russian biologist Zhores Medvedev publishes review stating that there are more than 300 theories of aging and the number is increasing.[26][100] | ||
| 1990 | Organization | The Gerontology Research Group is founded in Los Angeles by L. Stephen Coles. IT is a network of researchers from various disciplines with common interest in outer limits of aging and how to reach them in reasonably good health.[101] | United States | ||
| 1990-1995 | Scientific development | Concept introduction | The term negligible senescence is first used by professor Caleb Finch to describe organisms such as lobsters and hydras, which do not show symptoms of aging.[102] | ||
| 1991 | Scientific development | Theory | Applying a similar approach by Nesse in 1988 (see row), but to a larger set of wild mammalian populations, Promislow again concludes that there is detectable senescence in wild populations of animals.[2] | ||
| 1991 | Scientific development | Theory | Leonid A. Gavrilov and Natalia S. Gavrilova apply the principles of reliability theory to human biology, proposing a reliabity theory of aging which is based on the premise that humans are born in a highly defective state. According to the model, this is then made worse by environmental and mutational damage, and survival of the organism depends on redundancy.[103][104] | ||
| 1991 | Scientific development | Laboratory research | Leonard Hayflick describes an increase in cell degeneration and debris, resembling cell death.[8] | United States | |
| 1992 | Scientific development | Data science | The National Archive of Computerized Data on Aging (NACDA) publishes in the Internet the first 28 datasets related to aging. Gradually, the number of published datasets would surpass 1600. These datasets are available to any researcher around the world at no charge, so they can search in them for new patterns. The site also provides some tools to facilitate analysis.[105] | United States | |
| 1993 | Scientific development | Laboratory research | American molecular biologist Cynthia Kenyon discovers that a single-gene mutation (Daf-2) can double the lifespan of nematode Caenorhabditis elegans and that this can be reversed by a second mutation in daf-16m.[106][107] | United States | |
| 1994 | Literature | Leonard Hayflick publishes How and Why we Age, which elaborates on the difference between biological and chronological age and then explores on how understanding of aging has changed through history.[108] | |||
| 1994 | Scientific development | Laboratory research | Frisch and Francis report that loss of matrix attachment results in apoptosis. Since then, studies aimed at understanding the molecular mechanisms underlying the phenomenon coupled with advances in the field of apoptosis overall would help to elucidate several of the key players and to create hypotheses for how they are regulated.[55] | ||
| 1995 | Scientific development | Laboratory research | Detection of senescent cells using a cytochemical assay is first described.[109] Researchers discover that senescent cells express a β-galactosidase activity; and describe the “senescence-associated β galactosidase” (SA-βgal) biomarker, which conveniently identifies individual senescent cells in vitro and in vivo.[110] | ||
| 1995 | Scientific development | Laboratory research | The existence of senescence-associated beta-galactosidase is proposed by Dimri et al.[111] following the observation that when beta-galactosidase assays were carried out at pH 6.0, only cells in senescence state develop staining. They proposed a cytochemical assay based on production of a blue-dyed precipitate that results from the cleavage of the chromogenic substrate X-Gal, which stains blue when cleaved by galactosidase. Since then, even more specific quantitative assays were developed for its detection at pH 6.0.[112][113][114] | ||
| 1995 | Scientific development | Theory | The remodeling theory of aging is introduced.[99] It postulates that immunosenescence or 'immuno-remodelling' is a dynamic process involving both loss and gains of immune function.[115] | ||
| 1995 | Scientific development | Theory | Slade suggests that actuarial senescence may be negligible in small mammals.[2] | ||
| 1995 | Scientific development | Laboratory research | Research by by E. Wang shows that cellular senescence is associated with a reduced sensitivity to cell death.[8][116] | ||
| 1998 | Scientific development | Laboratory research | Scientists manage to extend, in a laboratory environment, the life of normal human cells beyond the Hayflick limit using telomerase.[117][118] | ||
| 1998 | Scientific development | Martinez observes that Hydra appear to grow older without showing signs of senescence. This is entirely consistent with the principle that the evolution of disposability of the soma presupposes the existence of a clear germ–soma distinction.[2] | |||
| 1998 | Scientific development | American academic Caleb Finch, and later other gerontologists and some demographers, argue that there is evidence of negligible senescence in natural populations of a number of species, from rockfish to tortoises.[2] | United States | ||
| 1999 | Organization (research institute) | The Buck Institute for Research on Aging is established as an independent biomedical research institute devoted solely to research on aging and age-related diseases.[119][120] | United States | ||
| 1999 | Literature | Aubrey de Grey publishes The Mitochondrial Free Radical Theory of Aging, which introduces the term "engineered negligible senescence".[121] | |||
| 2000 | Scienific development | Concept introduction | Sabina Sperandio and colleagues introduce the term "paraptosis". The group uses human insulin-like growth factor 1 receptor (IGF-1R) to stimulate cell death in 293T cells and mouse embryonic fibroblasts, observing distinct differences from other forms of cell death. They coin the term "paraptosis", derived from the Greek preposition para, meaning beside or related to, and apoptosis.[122][123] | United States | |
| 2003 | Scientific development | Laboratory research | Scientists report first evidence that aging of nematodes is regulated via mechanistic target ofrapamycin (mTOR) signaling.[124][125][126] | ||
| 2003 | Scientific development | Laboratory research | Andrzej Bartke creates a mouse that lived 1819 days (5 years without 7 days), while the maximum lifespan for this species is 1030–1070 days.[127] Translated to human standards, such longevity is equivalent to about 180 years.[128] | ||
| 2004 | Scientific development | Vaupel et al. describe two cases: one being a species that escapes from senescence which would exhibit the lack of such decline, referred to as "negligible senescence", and a second case in which a species shows even physiological improvement with age, which may be referred to as "negative senescence".[2] | |||
| 2004 | Scientific development | Data science | GenAge launches as the first curated database of genes related to human ageing.[129] It stores data of genes commonly altered during ageing, drawn from a microarray meta-analysis study, and the LongevityMap, a database of human genetic variants associated with longevity.[130] | ||
| 2004 | Scientific development | Theory | Reznick et al. suggest the need to develop ecologically oriented "derived" theories of ageing that include density- and condition-dependent interactions with senescent mortality. They state that density dependence affects aging because it interacts with the age profile of mortality and can lead to changes in resource availability. Also, like density dependence, they state that condition dependence can interact with extrinsic mortality and the rate of aging.[2] | ||
| 2005 | Scientific development | Kirkwood discovers that unicellular organisms such as yeasts and bacteria can exhibit forms of senescence.[2] | |||
| 2006 | Organization (research network) | Network Aging Research (Netzwerk Alternsforschung) is founded in Germany at Heidelberg University as a research network on the topic of aging. It supports researchers in multiple fields of aging, including humanists, natural- and medical scientists.[131] | Germany | ||
| 2006 | Literature (journal) | Clinical study | Peer-reviewed open access medical journal Clinical Interventions in Aging is founded, covering research in gerontology.[132] | ||
| 2006 | Scientific development | Laboratory research | Shinya Yamanaka and John Gurdon receive the Nobel Prize in Physiology or Medicine for their work on reprogramming mature cells into pluripotent cells.[133] Yamanaka is the first to produce induced stem cells (iSC) from somatic cells by the simultaneous action of several factors.[134][135][136] | ||
| 2007 | Scientific development | Laboratory research | Researchers manage to extend mouse lifespan via deletion of insulin receptor in the brain.[124][137] | ||
| 2007 | Scientific development | Study by Passos, Saretzki, and Ahmed et al. indicates that mitochondrial-derived reactive oxygen species can generate DNA damage, including single-stranded breaks in telomere regions, contributing to accelerated telomere shortening and premature senescence.[138][139] | United Kingdom (University of Newcastle) | ||
| 2007 | Scientific development | Laboratory research | Researchers report first evidence that a pharmacological agent (namely, metformin) at a certain dosage is capable to increase the lifespan of mice.[124][140] | ||
| 2008 | Scientific development | Concept introduction | The concept of senescence-associated secretory phenotype (SASP) is first established by Judith Campisi and her group, who first publish on the subject.[141] | United States | |
| 2008 | Scientific development | Coppe, Patil, Rodier et al. determine that senescent cells are known to secrete a number of proinflammatory molecules, a phenotype commonly known as the senescence-associated secretory phenotype (SASP).[138][142] | United States | ||
| 2008 | Organization | The Max Planck Institute for Biology of Ageing is founded. Its overall research aim is to obtain fundamental insights into the aging process and thus to pave the way towards healthier aging in humans.[143] | Germany | ||
| 2008 | Scientific development | Ricklefs, comparing age-related mortality patterns in birds in captivity and wild populations finds that the rate of senescence is similar to that in wild populations despite the absence of exogenous forces of mortality (i.e. predation, disease) in captivity.[2] | |||
| 2009 | Scientific development | Laboratory research | A group of three laboratories initiate a study of the effects of rapamycin on the life span of mice. After administering the agent at late ages, the team discovers a significant increase in maximum life span.[53] | ||
| 2009 | Recognition | Laboratory research | Elizabeth Blackburn, Carol Greider, and Jack Szostak are awarded the Nobel Prize in Physiology or Medicine "for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase."[144] These researchers are aware of the implications of their research on telomerase for the biology of aging, in particular Carol Greider who would become quite active in that field.[53] | ||
| 2009 | Scientific development | Laboratory research | Researchers report association of genetic variants in insulin/IGF1 signaling with human longevity.[124][145] | ||
| 2010 | Scientific development | Laboratory research | Harvard scientists reverse aging process in mice through reactivation of telomerase.[146] | United States | |
| 2010 | Scientific development | Laboratory research | Study by Passos, Nelson, Wang, et al. reports that reactive oxygen species produced by senescent cells are shown to lock senescent cells in a state of irreversible arrest by contributing to a persistent activation of a DNA damage response.[138][147] | ||
| 2010 | Organization (research network) | The European Network in Aging Studies is established.[148][149] It is a research network that connects researchers with the purpose to facilitate sustainable international and multi-disciplinary collaboration among all researchers interested in the study of cultural aging.[150] | Europe | ||
| 2011 | Scientific development | Laboratory research | Study by Baker, Wijshake, and Tchkonia et al.[151] (confirmed later by Baker, Childs, and Durik et al. in 2016[152]) reports that senescent cells accumulate with age and at sites of pathology, where they are shown to drive tissue deterioration.[138] | United States (Mayo Clinic College of Medicine and Science, Rochester, Minnesota) | |
| 2012 | Scientific development | Laboratory research | Researchers from the Institute of Molecular Oncology (IFOM) in Milan and Molecular Genetics of the National Research Council (IGM-CNR) in Pavia identify for the first time a class of non-coding RNAs, called “DNA Damage Response RNAs (DDRNAs)”, laying the foundation for the future advances in cellular aging.[153] | Italy | |
| 2012 | Scientific development | Laboratory research | Research by Nelson et al. shows that the bystander effect of senescent cells negatively affects non‐senescent cells via reactive oxygen species (ROS). The researchers also show that a paracrine effect of senescent cells can damage DNA.[8][154] | United Kingdom (Newcastle University) | |
| 2013 (January) | Organization | The North American Network in Aging Studies (NANAS) is established with the ultimate purpose to improve the health, care, and quality of life for people aging into old age.[148] | United States | ||
| 2013 | Scientific development | Laboratory research | A group of scientists define nine hallmarks of aging that are common between organisms with emphasis on mammals:
|
||
| 2013 | Scientific development | Theory | Silvertown suggests that the ways in which senescence is thought to evolve precludes any common mechanism of decline.[2] | ||
| 2013 | Scientific development | Laboratory research | Nussey et al. conduct literature survey, and find clear evidence of actuarial senescence (senescence in survival) in large mammalian species (i.e. ungulates, marine mammals and primates).[2] | ||
| 2014 | Scientific development | Laboratory research | Researchers report first evidence that pharmacological activation of SIRT1 extends lifespan in mice and improves their health.[124][156][157] | ||
| 2014 | Scientific development | Laboratory research | Study by Correia-Melo, Hewitt, and Passos indicates that senescence is a multilayered process which involves dramatic time-dependent changes in gene expression, epigenetic and metabolic profile.[138] | ||
| 2015 | Scientific development | Laboratory research | Study by Madeo, Zimmermann, Maiuri and Kroemer determines that autophagy plays an important role during senescence through the elimination of old or unneeded materials.[158] | ||
| 2015 | Scientific development | Laboratory research | Study by Xu, Palmer, and Ding et al.[159] (later confirmed by Baker, Childs, and Durik et al.[160]) indicates that the accumulation of senescent cells in multiple tissues contributes causally to age-related tissue dysfunction.[138] | United States (Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota) | |
| 2016 | Scientific development | Laboratory research | Scientists at Newcastle University demonstrate for the first time that mitochondria are major triggers of cell aging, after having found that when mitochondria are eliminated from aging cells, the latter become much more similar to younger cells. This discovery advances a step closer to developing therapies to counteract the aging of cells, by targeting mitochondria.[161][162][163] | United Kingdom (Newcastle University) | |
| 2016 | Scientific development | Laboratory research | Researchers find that the replenishment of nicotinamide adenine dinucleotide (NAD+) in the organism of mice through precursor molecules improves the functioning of mitochondria and stem cells, and also leads to an increase in their lifespan.[124][164] One of these NAD+ precursor molecules is nicotinamide mononucleotide NMN.[165][166] | ||
| 2016 | Scientific development | Laboratory research | Researchers demonstrate that a combination of longevity associated drugs can additively extend lifespan in mice.[124][167] | ||
| 2016 | Scientific development | Laboratory research | Study by Childs, Baker, and Wijshake et al. concludes that senescent intimal foam cells are deleterious at all stages of atherosclerosis.[138][168] | ||
| 2016 | Scientific development | Laboratory research | Correia-Melo, Marques, Anderson et al. determine that mitochondria are the powerhouses of cells and are essential for the development of cellular senescence.[138][169] | ||
| 2017 | Scientific development | Laboratory research | Researchers discover that a naturally occurring polymorphism in human signaling pathways is in some cases associated with health and longevity. It is also detected that, the same as in mice, this association can depend on the gender (it can be observed for one gender but not for another). This indicates that by correctly influencing these pathways, it is theoretically possible to alter lifespan and healthspan in humans.[124][170] | ||
| 2017 | Scientific development | Laboratory research | Study by Jeon, Kim, and Laberge et al. reports that senescent cells accumulate in many vertebrate tissues with age and contribute to age-related pathologies, presumably through their secretion of factors contributing to the senescence-associated secretory phenotype,[171] the latter which defines the ability of senescent cells to express and secrete a variety of extracellular modulators that includes cytokines, chemokines, proteases, growth factors and bioactive lipids.[172] | ||
| 2017 (December) | Scientific development | Laboratory research | Study shows that preventing wrinkles could be as easy as expressing a protein called FKBP1b.[173] | ||
| 2018 (January) | Scientific development | Laboratory research | Researchers at the University of Texas Health Science Center (UTHealth) in Houston report a connection between accelerated epigenetic aging and bipolar disorder. The results could explain why people suffering from bipolar disorder are more likely to die from age-related diseases.[174] | United States | |
| 2018 | Policy | The World Health Organization includes in the international classification of diseases ICD-11 a special additional code signaling the relationship of a disease with age. As a result, after the final approval of the ICD-11 in May 2019, aging would begin to be officially recognized as a fundamental factor that increases the risk of diseases, the severity of their course and the difficulty of treatment.[175][176][177][178][179] | |||
| 2018 | Scientific development | Laboratory research | Researchers identify naked mole-rats as the first mammal to defy the Gompertz–Makeham law of mortality, and achieve negligible senescence. It would be speculated however that this may be simply a "time-stretching" effect primarily due to their very slow (and cold-blooded and hypoxic) metabolism.[180][181] | ||
| 2019 | Scientific development | Laboratory research | Researchers show that many major features of cellular senescence, such as the pro‐inflammatory phenotype, are dependent on the stable cell cycle arrest.[182][8] | ||
| 2019 | Scientific development | Laboratory research | Scientists manage to extend the average lifespan of mice by breeding them using embryonic stem cells with extra-long telomeres. The finding is significant as no genetic modification is conducted.[183][184][185] | ||
| 2019 (December 9) | Scientific development | Laboratory research | Researchers at the Pasteur Institute identify the CSB protein, whose absence or dysfunction causes early aging, among other afflictions, in patients with Cockayne syndrome.[186] | France | |
| 2020 (July) | Scientific development | Laboratory research | Scientists, using public biological data on 1.75 million people with known lifespans overall, identify 10 genomic loci which appear to intrinsically influence healthspan, lifespan, and longevity – of which half have not been reported previously at genome-wide significance and most being associated with cardiovascular disease – and identify haem metabolism as a promising candidate for further research within the field. Their study suggests that high levels of iron in the blood likely reduce, and genes involved in metabolising iron likely increase healthy years of life in humans.[187] | United Kingdom | |
| 2020 (December) | Scientific development | Laboratory research | Researchers at University of California, San Francisco report on an experimental drug called ISRIB, which restores memory function months after traumatic brain injury (TBI) in mice.[188] | United States |
Visual and numerical data
Mentions on Google Scholar
The table below summarizes per-year mentions of senescence–related topics (entries without quotation marks) on Google Scholar as of May 13, 2021.
| Year | Senescence | Aging | Longevity | Life extension |
|---|---|---|---|---|
| 1980 | 2,840 | 24,300 | 7,200 | 48,900 |
| 1985 | 3,760 | 36,300 | 9,210 | 43,400 |
| 1990 | 5,840 | 69,200 | 13,400 | 101,000 |
| 1995 | 8,150 | 114,000 | 18,300 | 131,000 |
| 2000 | 12,200 | 237,000 | 33,600 | 269,000 |
| 2002 | 13,400 | 302,000 | 41,600 | 299,000 |
| 2004 | 16,600 | 403,000 | 51,300 | 296,000 |
| 2006 | 21,500 | 470,000 | 62,900 | 341,000 |
| 2008 | 26,800 | 481,000 | 74,500 | 364,000 |
| 2010 | 33,500 | 587,000 | 85,400 | 397,000 |
| 2012 | 43,500 | 712,000 | 109,000 | 409,000 |
| 2014 | 47,900 | 653,000 | 103,000 | 366,000 |
| 2016 | 47,200 | 466,000 | 88,800 | 298,000 |
| 2017 | 45,300 | 441,000 | 81,500 | 254,000 |
| 2018 | 43,800 | 297,000 | 74,600 | 194,000 |
| 2019 | 39,500 | 201,000 | 59,400 | 148,000 |
| 2020 | 34,800 | 130,000 | 44,200 | 103,000 |
Google Trends
The comparative chart below shows Google Trends data for Senescence (Topic), Cellular senescence (Topic) and Negligible senescence (Topic), from January 2004 to April 021, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[189]
Google Ngram Viewer
The chart below shows Google Ngram Viewer data for Senescence research, from 1950 to 2019.[190]
Wikipedia Views
The chart below shows pageviews of the English Wikipedia article Senescence, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to March 2021.[191]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by User:Sebastian.
Funding information for this timeline is available.
What the timeline is still missing
- Suggestions for expansion (from Vipul); don't limit yourself just to these:
- Probably we should add more column(s) to classify by the theory or approach that the row is about. These columns may be blank for some rows.✔/✘
- There should be more context on individual rows, including possibly some context that explains the significance and current standing.✔/✘ The idea is that readers reading it should have a sense of the ups and downs of specific theories in the marketplace of ideas.✔/✘
- Added 2021-08-28: Big picture would also benefit from some summary around the different theories.
- Maybe some charts of things based on Google Scholar✔, research budgets✘, etc.
- Suggestions for expansion (from Vipul); don't limit yourself just to these:
Timeline update strategy
See also
- Timeline of life extension
- Timeline of CRISPR
- Timeline of Alzheimer's disease
- Timeline of calorie restriction
External links
References
- ↑ 1.0 1.1 1.2 1.3 Andrea Grignolio; Claudio Franceschi. "History of Research into Aging/Senescence". eLS. doi:10.1002/9780470015902.a0023955.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 Shefferson, Richard P.; Jones, Owen R.; Salguero-Gómez, Roberto (23 February 2017). The Evolution of Senescence in the Tree of Life. Cambridge University Press. ISBN 978-1-107-07850-5.
- ↑ 3.0 3.1 3.2 3.3 A History of Life-Extensionism In The Twentieth Century. Rison Lezion, Israel: Longevity History. 2014. ISBN 1500818577.
- ↑ "Life Expectancy".
- ↑ 5.0 5.1 5.2 5.3 Jin, Kunlin (2010-08-01). "Modern Biological Theories of Aging". Aging and Disease. 1 (2): 72–74. ISSN 2152-5250.
- ↑ Fletcher, James Rupert (December 2020). "Anti-aging technoscience & the biologization of cumulative inequality: Affinities in the biopolitics of successful aging". Journal of Aging Studies. 55: 100899. ISSN 0890-4065. doi:10.1016/j.jaging.2020.100899.
- ↑ Binstock, Robert H. (February 2003). "The War on "Anti-Aging Medicine"". The Gerontologist. 43 (1): 4–14. doi:10.1093/geront/43.1.4.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Ogrodnik, Mikolaj. "Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo". Aging Cell. 20 (4). doi:10.1111/acel.13338.
- ↑ 9.0 9.1 Handbook of the biology of aging (6th ed.). Amsterdam: Elsevier Academic Press. 2006. ISBN 9780120883875.
- ↑ Fried, Linda P.; Rowe, John W. (2020-10-01). "Health in Aging — Past, Present, and Future". New England Journal of Medicine. 383 (14): 1293–1296. doi:10.1056/NEJMp2016814.
- ↑ Zhavoronkov, Alexander; Bhupinder, Bhullar (4 October 2015). "Classifying aging as a disease in the context of ICD-11". Frontiers in Genetics. 6: 326. PMC 4631811
 . PMID 26583032. doi:10.3389/fgene.2015.00326.
. PMID 26583032. doi:10.3389/fgene.2015.00326.
- ↑ Stambler, Ilia (1 October 2017). "Recognizing Degenerative Aging as a Treatable Medical Condition: Methodology and Policy". Aging and Disease. 8 (5): 583–589. PMC 5614323
 . PMID 28966803. doi:10.14336/AD.2017.0130
. PMID 28966803. doi:10.14336/AD.2017.0130 .
.
- ↑ "Opening the door to treating ageing as a disease". The Lancet Diabetes & Endocrinology. 6 (8): 587. 1 August 2018. doi:10.1016/S2213-8587(18)30214-6.
- ↑ Calimport, Stuart; et al. (1 October 2019). "To help aging populations, classify organismal senescence". Science. 366 (6465): 576–578. doi:10.1126/science.aay7319.
- ↑ Khaltourina, Daria; Matveyev, Yuri; Alekseev, Aleksey; Cortese, Franco; Ioviţă, Anca (July 2020). "Aging Fits the Disease Criteria of the International Classification of Diseases". Mechanisms of Ageing and Development. 189: 111230. doi:10.1016/j.mad.2020.111230.
- ↑ 16.0 16.1 16.2 Daniel Fabian; Thomas Flatt. "The Evolution of Aging". Nature.
- ↑ Gompertz, B. (1825). "On the Nature of the Function Expressive of the Law of Human Mortality, and on a New Mode of Determining the Value of Life Contingencies". Philosophical Transactions of the Royal Society. 115: 513–585. doi:10.1098/rstl.1825.0026.
- ↑ Leonid A. Gavrilov & Natalia S. Gavrilova (1991) The Biology of Life Span: A Quantitative Approach. New York: Harwood Academic Publisher, ISBN 3-7186-4983-7
- ↑ "APA Dictionary of Psychology". dictionary.apa.org. Retrieved 23 December 2021.
- ↑ "Biological Aging Theory - Frequently asked Questions and Answers".
- ↑ "A Weismann".
- ↑ Harris DK (1988). Dictionary of Gerontology
 . New York: Greenwood Press. p. 80. ISBN 9780313252877.
. New York: Greenwood Press. p. 80. ISBN 9780313252877.
- ↑ Metchnikoff E (1903). The Nature of Man: Studies in Optimistic Philosophy. Translated by Mitchell PC. New York and London: G.P. Putnam's Sons. OCLC 173625.
- ↑ Grignolio A, Franceschi C (15 June 2012). "History of Research into Ageing/Senescence". eLS. American Cancer Society. ISBN 978-0470016176. doi:10.1002/9780470015902.a0023955.
- ↑ 25.0 25.1 25.2 Dazhong, Yin; Chen, Keji. "The essential mechanisms of aging: Irreparabe damage accumulation of biochemical side-reactions".
- ↑ 26.00 26.01 26.02 26.03 26.04 26.05 26.06 26.07 26.08 26.09 26.10 26.11 26.12 26.13 26.14 26.15 26.16 26.17 26.18 26.19 26.20 26.21 Medvedev, Zhores A. (August 1990). "AN ATTEMPT AT A RATIONAL CLASSIFICATION OF THEORIES OF AGING". Biological Reviews. 65 (3): 375–398. doi:10.1111/j.1469-185x.1990.tb01428.x.
- ↑ Michael Ristow; Kathrin Schmeisser (2014). "Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS)". Dose Response. 12: 288–341. PMC 4036400
 . PMID 24910588. doi:10.2203/dose-response.13-035.Ristow.
. PMID 24910588. doi:10.2203/dose-response.13-035.Ristow.
- ↑ Rubner, M. (1908). Das Problem det Lebensdaur und seiner beziehunger zum Wachstum und Ernarnhung. Munich: Oldenberg.
- ↑ Stark, Gavin; Pincheira‐Donoso, Daniel; Meiri, Shai (May 2020). "No evidence for the 'rate‐of‐living' theory across the tetrapod tree of life". Global Ecology and Biogeography. 29 (5): 857–884. doi:10.1111/geb.13069.
- ↑ Hulbert, A. J.; Pamplona, Reinald; Buffenstein, Rochelle; Buttemer, W. A. (1 October 2007). "Life and Death: Metabolic Rate, Membrane Composition, and Life Span of Animals". Physiological Reviews. pp. 1175–1213. doi:10.1152/physrev.00047.2006. Retrieved 7 October 2021.
- ↑ Olshansky, S. J.; Rattan, Suresh IS (25 July 2009). "What Determines Longevity: Metabolic Rate or Stability?". Discovery Medicine. pp. 359–362.
- ↑ Magalhães, João Pedro de; Costa, Joana; Church, George M. (1 February 2007). "An Analysis of the Relationship Between Metabolism, Developmental Schedules, and Longevity Using Phylogenetic Independent Contrasts". The Journals of Gerontology: Series A. 62 (2): 149–160. doi:10.1093/gerona/62.2.149.
- ↑ "Cross linking theory of aging" (PDF).
- ↑ 34.0 34.1 David Costantini. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology. p. 306.
- ↑ "Theory 5: Rate of Living Theory | Biology of Aging". courses.lumenlearning.com. Retrieved 7 October 2021.
- ↑ Stambler, Ilia (29 August 2014). "reference No. 438". A History of Life-Extensionism in the Twentieth Century. Longevity History. p. 540. ISBN 978-1500818579.
- ↑ Fossel, Michael. The Telomerase Revolution: The Enzyme That Holds the Key to Human Aging.
- ↑ Stambler, Ilia (29 August 2014). "Institutionalization of gerontology – Max Bürger". A History of Life-Extensionism in the Twentieth Century. Longevity History. p. 540. ISBN 978-1500818579.
- ↑ Ilia Stambler (January 2019). "History of Life-Extensionism". Encyclopedia of Biomedical Gerontology: 228–237. ISBN 9780128012383. doi:10.1016/B978-0-12-801238-3.11331-5. Retrieved 5 May 2021.
- ↑ Старость. (Труды конференции по проблеме генеза старости и профилактики преждевременного стрения организма) [Old age. (Proceedings of the conference on the problem of the genesis of old age and the prevention of premature abrasion of the body)] (in русский). Kiev: UkrSSR Academy of Sciences Publishing House. 1939. p. 490.
- ↑ "The British Society For Research On Aging • scientia.global". scientia.global. 2017-02-12. Retrieved 8 June 2021.
- ↑ "HOME PAGE". BSRA. Retrieved 17 June 2021.
- ↑ Diggs, Jessica (2008). "The Cross‐Linkage Theory of Aging". Encyclopedia of Aging and Public Health. Springer US: 250–252. doi:10.1007/978-0-387-33754-8_112.
- ↑ "Theory 7: Cross-linkage Theory | Biology of Aging". courses.lumenlearning.com. Retrieved 7 October 2021.
- ↑ Gerschman, R.; Gilbert, D. L.; Nye, S. W.; Dwyer, P.; Fenn, W. O. (1954-05-07). "Oxygen Poisoning and X-irradiation: A Mechanism in Common". Science. 119 (3097): 623–626. doi:10.1126/science.119.3097.623.
- ↑ "The Gerontological Society of America". Oxford Academic. Retrieved 2 July 2021.
- ↑ "Journal of Gerontology | Oxford Academic". OUP Academic. Retrieved 2 July 2021.
- ↑ "About the IAG". www.sfu.ca. Retrieved 12 July 2021.
- ↑ Ward Dean (22 March 2012). "Neuroendocrine Theory of Aging". warddeanmd.com. Retrieved 12 July 2021.
- ↑ Dilman, V.M. (June 1971). "AGE-ASSOCIATED ELEVATION OF HYPOTHALAMIC THRESHOLD TO FEEDBACK CONTROL, AND ITS ROLE IN DEVELOPMENT, AGEING, AND DISEASE". The Lancet. 297 (7711): 1211–1219. doi:10.1016/s0140-6736(71)91721-1.
- ↑ Dilman, V.M.; Revskoy, S.Y.; Golubev, A.G. (1986). "Neuroendocrine-Ontogenetic Mechanism of Aging: Toward An Integrated Theory of Aging". International Review of Neurobiology. 28: 89–156. doi:10.1016/S0074-7742(08)60107-5.
- ↑ Bland, Jeffrey S. "Age as a Modifiable Risk Factor for Chronic Disease". Integrative Medicine: A Clinician's Journal. 17 (4): 16–19. ISSN 1546-993X.
- ↑ 53.0 53.1 53.2 Martin, George M. (November 2011). "The biology of aging: 1985–2010 and beyond". The FASEB Journal. 25 (11): 3756–3762. ISSN 0892-6638. doi:10.1096/fj.11-1102.ufm.
- ↑ Gladyshev, Vadim N. (February 2014). "The Free Radical Theory of Aging Is Dead. Long Live the Damage Theory!". Antioxidants & Redox Signaling. 20 (4): 727–731. doi:10.1089/ars.2013.5228.
- ↑ 55.0 55.1 55.2 Gewirtz, David A.; Holt, Shawn E.; Grant, Steven (23 October 2007). Apoptosis, Senescence and Cancer. Springer Science & Business Media. ISBN 978-1-59745-221-2.
- ↑ Williams, G.C. (1957). "Pleiotropy, natural selection and the evolution of senescence" (PDF). Evolution. 11 (4): 398–411. JSTOR 2406060. doi:10.2307/2406060. Paper in which Williams describes his theory of antagonistic pleiotropy.
- ↑ LAMBREMQNT, EDWARD NELSON. "A STUDY OF THE BIOCHEMISTRY AND PHYSIOLOGICAL AGING OF TWO PHOSPHATASES OF THE MOSQUITO, AEDES AEGYPTI".
- ↑ "The Baltimore Longitudinal Syudy of Aging (BLSA)". clinicaltrials.gov. clinicaltrials.gov. 2021-05-01. Retrieved 12 June 2021.
- ↑ Boniewska-Bernacka E (2016). "Selected Theories of Aging" (PDF). Higher School's Pulse. 10: 36–39.
- ↑ "How Your Immune System Might Be at the Heart of How You Age". Verywell Health. Retrieved 17 August 2021.
- ↑ "Will the Hayflick limit keep us from living forever?".
- ↑ Hayflick L, Moorhead PS (1961). "The serial cultivation of human diploid cell strains". Exp Cell Res. 25 (3): 585–621. PMID 13905658. doi:10.1016/0014-4827(61)90192-6.
- ↑ Hayflick L. (1965). "The limited in vitro lifetime of human diploid cell strains". Exp. Cell Res. 37 (3): 614–636. PMID 14315085. doi:10.1016/0014-4827(65)90211-9.
- ↑ Diggs, Jessica (2008). "The Error Catastrophe (Accumulation) Theory of Aging". Encyclopedia of Aging and Public Health: 329–330. doi:10.1007/978-0-387-33754-8_162.
- ↑ Lange, Jean; Grossman, Sheila. "Theories of aging" (PDF). samples.jbpub.com.
- ↑ 66.0 66.1 66.2 66.3 Tissenbaum, Heidi A. (2015-01-30). "Using C. elegans for aging research". Invertebrate Reproduction & Development. 59 (sup1): 59–63. ISSN 0792-4259. doi:10.1080/07924259.2014.940470.
- ↑ Lockshin RA, Williams CM (1964). "Programmed cell death—II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths". Journal of Insect Physiology. 10 (4): 643–649. doi:10.1016/0022-1910(64)90034-4.
- ↑ "Programmed Cell Death - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 7 October 2021.
- ↑ Weinert, Brian T.; Timiras, Poala S. (October 2003). "Invited Review: Theories of aging". Journal of Applied Physiology. 95 (4): 1706–1716. doi:10.1152/japplphysiol.00288.2003.
- ↑ Hayflick, L. (March 1965). "The limited in vitro lifetime of human diploid cell strains". Experimental Cell Research. 37 (3): 614–636. doi:10.1016/0014-4827(65)90211-9.
- ↑ Berdyshev, G; Korotaev, G; Boiarskikh, G; Vaniushin, B (1967). "Nucleotide composition of DNA and RNA from somatic tissues of humpback and its changes during spawning". Biokhimiia. 31: 88–993.
- ↑ Horvath S (2013). "DNA methylation age of human tissues and cell types". Genome Biology. 14 (R115): R115. PMC 4015143
 . PMID 24138928. doi:10.1186/gb-2013-14-10-r115.
. PMID 24138928. doi:10.1186/gb-2013-14-10-r115.
- ↑ Alexander P (1967). "The role of DNA lesions in the processes leading to aging in mice". Symp. Soc. Exp. Biol. 21: 29–50. PMID 4860956.
- ↑ Bernstein C, Bernstein H (1991). Aging, Sex, and DNA Repair. San Diego CA: Academic Press. ISBN 0123960037.
- ↑ Ames BN, Gold LS (1991). "Endogenous mutagens and the causes of aging and cancer". Mutat. Res. 250 (1-2): 3–16. PMID 1944345. doi:10.1016/0027-5107(91)90157-j.
- ↑ Diggs, Jessica (2008). "Autoimmune Theory of Aging". Encyclopedia of Aging and Public Health: 143–144. doi:10.1007/978-0-387-33754-8_46.
- ↑ "Theory 3: Autoimmune Theory | Biology of Aging". courses.lumenlearning.com. Retrieved 7 October 2021.
- ↑ "General Adaptation Syndrome: Your Body's Response to Stress". Healthline. 1 May 2017. Retrieved 23 December 2021.
- ↑ Ocaklı, Burcu Özdemir (2019). "American Aging Association". Encyclopedia of Gerontology and Population Aging. Springer International Publishing. pp. 1–5. doi:10.1007/978-3-319-69892-2_218-1. Retrieved 15 June 2021.
- ↑ Ofahengaue Vakalahi, Halaevalu F.; Simpson, Gaynell M.; Giunta, Nancy. The Collective Spirit of Aging Across Cultures. p. 20. Retrieved 21 December 2016.
- ↑ "National Institute of Aging".
- ↑ Robinson, A. B. (1974-03-01). "Evolution and the Distribution of Glutaminyl and Asparaginyl Residues in Proteins". Proceedings of the National Academy of Sciences. 71 (3): 885–888. doi:10.1073/pnas.71.3.885.
- ↑ "ELIZABETH BLACKBURN: TELOMERES AND TELOMERASE".
- ↑ Blackburn AM; Gall, Joseph G. (March 1978). "A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena". J. Mol. Biol. 120 (1): 33–53. PMID 642006. doi:10.1016/0022-2836(78)90294-2.
- ↑ "The 2009 Nobel Prize in Physiology or Medicine - Press Release". Nobelprize.org. 2009-10-05. Retrieved 2012-06-12.
- ↑ "Unravelling the secret of aging". COSMOS: The Science of Everything. October 5, 2009. Archived from the original on January 14, 2015.
- ↑ Goldsmith, Theodore. The Evolution of Aging: How New Theories Will Change the Future of Medicine. p. 48.
- ↑ Blagosklonny, Mikhail V. (29 December 2010). "Why the disposable soma theory cannot explain why women live longer and why we age". Aging. 2 (12): 884–887. doi:10.18632/aging.100253.
- ↑ Sotola, Lukas (2016). "Disposable Soma Theory". Encyclopedia of Evolutionary Psychological Science: 1–4. doi:10.1007/978-3-319-16999-6_2367-1.
- ↑ "БИОГЕНЕЗ МИТОХОНДРИЙ ПРИ ДИФФЕРЕНЦИАЦИИ И СТАРЕНИИ КЛЕТОК" (PDF). aiexandr2010.narod.ru. Retrieved 10 June 2021.
- ↑ "Mitochondrial Theory of Aging — IVAO". www.ivao.com. Retrieved 10 June 2021.
- ↑ Das, R; Kanungo, MS (June 1980). "In vitro phosphorylation of chromosomal proteins of the brain of rats of various ages & its modulation by epinephrine.". Indian journal of biochemistry & biophysics. 17 (3): 217–21. PMID 7450808.
- ↑ "Alliance for Aging Research". www.idealist.org. Retrieved 8 June 2021.
- ↑ Gavrilov LA, Gavrilova NS (1986). Skulachev WP, ed. Биология продолжительности жизни: Количественные аспекты [Biology of Life Span: Quantitative Aspects] (in русский) (1st ed.). Moscow: Nauka. p. 167.
- ↑ Gavrilov LA, Gavrilova NS (1991). Skulachev VP, ed. Biology of Life Span: A Quantitative Approach (1st ed.). New York: Chur. p. 385. ISBN 978-3718649839.
- ↑ Gavrilov LA, Gavrilova NS (December 2001). "The reliability theory of aging and longevity". Journal of Theoretical Biology. 213 (4): 527–45. PMID 11742523. doi:10.1006/jtbi.2001.2430.
- ↑ A.J.S. Rayl (13 May 2002). "Aging, in Theory: A Personal Pursuit. Do body system redundancies hold the key?" (PDF). The Scientist. 16 (10): 20.
- ↑ Stanulis-Praeger, B. M. (March 1987). "Cellular senescence revisited: a review". Mechanisms of Aging and Development. 38 (1): 1–48. ISSN 0047-6374. doi:10.1016/0047-6374(87)90109-6.
- ↑ 99.0 99.1 Franceschi, C; Valensin, S; Bonafè, M; Paolisso, G; Yashin, A.I; Monti, D; De Benedictis, G (September 2000). "The network and the remodeling theories of aging: historical background and new perspectives". Experimental Gerontology. 35 (6-7): 879–896. doi:10.1016/S0531-5565(00)00172-8.
- ↑ Viña, Jose; Borrás, Consuelo; Miquel, Jaime (2007). "Theories of ageing". IUBMB Life. 59 (4): 249–254. doi:10.1080/15216540601178067.
- ↑ options, Show more sharing; URLCopied!, Copy Link (5 December 2014). "L. Stephen Coles dies at 73; studied extreme aging in humans". Los Angeles Times. Retrieved 13 July 2021.
- ↑ Greg Critser. Eternity Soup: Inside the Quest to End Aging.
- ↑ A. J. S. Rayl (May 13, 2002). "Aging, in Theory: A Personal Pursuit". The Scientist.
- ↑ Leonid A. Gavrilov, Natalia S. Gavrilova; V.P. Skulachev (ed.); John and Liliya Payne (trans.) (1991). The Biology of Life Span: A Quantitative Approach. Chur; New York: Harwood Academic Publishers. ISBN 9783718649839.
- ↑ "About Us". NACDA. Retrieved 7 May 2021.
- ↑ "Finding the Fountain of Youth / Where will UCSF biochemist Cynthia Kenyon's age-bending experiments with worms lead us?".
- ↑ Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993). "A C. elegans mutant that lives twice as long as wild type". Nature. 366 (6454): 461–464. PMID 8247153. doi:10.1038/366461a0.
- ↑ Zane Bartlett. "A History of Cellular Senescence and Its Relation to Stem Cells in the Twentieth and Twenty-First Centuries" (PDF). Retrieved 4 August 2016.
- ↑ "Senescence Associated β-galactosidase Staining". Retrieved 20 August 2016.
- ↑ Itahana, Koji; Itahana, Yoko; Dimri, Goberdhan P. (2013). "Colorimetric Detection of Senescence-Associated β Galactosidase". Methods in molecular biology (Clifton, N.J.). 965: 143–156. ISSN 1064-3745. doi:10.1007/978-1-62703-239-1_8.
- ↑ Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O (September 1995). "A biomarker that identifies senescent human cells in culture and in aging skin in vivo". Proc. Natl. Acad. Sci. U.S.A. 92 (20): 9363–7. PMC 40985
 . PMID 7568133. doi:10.1073/pnas.92.20.9363.
. PMID 7568133. doi:10.1073/pnas.92.20.9363.
- ↑ Bassaneze V, Miyakawa AA, Krieger JE (January 2008). "A quantitative chemiluminescent method for studying replicative and stress-induced premature senescence in cell cultures". Anal. Biochem. 372 (2): 198–203. PMID 17920029. doi:10.1016/j.ab.2007.08.016.
- ↑ Gary RK, Kindell SM (August 2005). "Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts". Anal. Biochem. 343 (2): 329–34. PMID 16004951. doi:10.1016/j.ab.2005.06.003.
- ↑ Itahana K, Campisi J, Dimri GP (2007). Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay
 . Methods in Molecular Biology. 371. pp. 21–31. ISBN 978-1-58829-658-0. PMID 17634571. doi:10.1007/978-1-59745-361-5_3.
. Methods in Molecular Biology. 371. pp. 21–31. ISBN 978-1-58829-658-0. PMID 17634571. doi:10.1007/978-1-59745-361-5_3.
- ↑ Franceschi, C; Valensin, S; Bonafè, M; Paolisso, G; Yashin, A.I; Monti, D; De Benedictis, G (September 2000). "The network and the remodeling theories of aging: historical background and new perspectives". Experimental Gerontology. 35 (6-7): 879–896. doi:10.1016/S0531-5565(00)00172-8.
- ↑ Wang, E. (1995-06-01). "Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved". Cancer Research. 55 (11): 2284–2292. ISSN 0008-5472.
- ↑ Varela, E; Blasco, M A (March 2010). "2009 Nobel Prize in Physiology or Medicine: telomeres and telomerase". Oncogene. 29 (11): 1561–1565. doi:10.1038/onc.2010.15.
- ↑ Bodnar, A. G. (16 January 1998). "Extension of Life-Span by Introduction of Telomerase into Normal Human Cells". Science. 279 (5349): 349–352. doi:10.1126/science.279.5349.349.
- ↑ "About". BUCK. Retrieved 9 June 2021.
- ↑ "Buck Institute for Research on Aging". www.omicsonline.org. Retrieved 9 June 2021.
- ↑ de Grey, Aubrey (November 2003). The Mitochondrial Free Radical Theory of Aging. Austin, Texas: Landes Bioscience.
- ↑ Sperandio, S; de Belle, I; Bredesen, DE (Dec 19, 2000). "An alternative, nonapoptotic form of programmed cell death.". Proceedings of the National Academy of Sciences of the United States of America. 97 (26): 14376–81. PMC 18926
 . PMID 11121041. doi:10.1073/pnas.97.26.14376.
. PMID 11121041. doi:10.1073/pnas.97.26.14376.
- ↑ "Paraptosis - Oxford Dictionaries".
- ↑ 124.0 124.1 124.2 124.3 124.4 124.5 124.6 124.7 Zainabadi, Kayvan (April 2018). "A brief history of modern aging research". Experimental Gerontology. 104: 35–42. doi:10.1016/j.exger.2018.01.018.
- ↑ McCay CM, Crowell M (October 1934). "Prolonging the Life Span". The Scientific Monthly. 39 (5): 405–414. JSTOR 15813.
- ↑ Vellai, Tibor; Takacs-Vellai, Krisztina; Zhang, Yue; Kovacs, Attila L.; Orosz, László; Müller, Fritz (December 2003). "Influence of TOR kinase on lifespan in C. elegans". Nature. 426 (6967): 620–620. doi:10.1038/426620a.
- ↑ В.Е. Чернилевский, В.Н. Крутько (2000). "История изучения средств продления жизни" [History of studying the means of extending life] (in русский). National Gerontology Center (of Russia). Retrieved 5 May 2021.
- ↑ "Battle for 'old mouse' prize". news.bbc.co.uk. 4 September 2003. Retrieved 9 July 2021.
- ↑ Magalhães, João Pedro de; Toussaint, Olivier (2004). "GenAge: a genomic and proteomic network map of human ageing". FEBS Letters. 571 (1–3): 243–247. ISSN 1873-3468. doi:10.1016/j.febslet.2004.07.006
 . Retrieved 26 June 2021.
. Retrieved 26 June 2021.
- ↑ "GenAge: The Ageing Gene Database". genomics.senescence.info. Retrieved 17 August 2021.
- ↑ "Das Netzwerk AlternsfoRschung (NAR) der Ruprecht-Karls-Universität Heidelberg (PDF-E-Book). Psychotherapie im Alter 2010, 7(3), 405-410". Psychosozial-Verlag (in Deutsch). Retrieved 9 June 2021.
- ↑ Press, Dove. "Clinical Interventions in Aging - Dove Press Open Access Publisher". www.dovepress.com. Retrieved 2 July 2021.
- ↑ "The Nobel Prize in Physiology or Medicine 2012". NobelPrize.org. Retrieved 9 July 2021.
- ↑ Takahashi, Kazutoshi; Yamanaka, Shinya (August 2006). "Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors". Cell. 126 (4): 663–676. doi:10.1016/j.cell.2006.07.024.
- ↑ Takahashi, Kazutoshi; Tanabe, Koji; Ohnuki, Mari; Narita, Megumi; Ichisaka, Tomoko; Tomoda, Kiichiro; Yamanaka, Shinya (November 2007). "Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors". Cell. 131 (5): 861–872. doi:10.1016/j.cell.2007.11.019.
- ↑ Okita, Keisuke; Ichisaka, Tomoko; Yamanaka, Shinya (July 2007). "Generation of germline-competent induced pluripotent stem cells". Nature. 448 (7151): 313–317. doi:10.1038/nature05934.
- ↑ Taguchi, A.; Wartschow, L. M.; White, M. F. (20 July 2007). "Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis". Science. 317 (5836): 369–372. doi:10.1126/science.1142179.
- ↑ 138.0 138.1 138.2 138.3 138.4 138.5 138.6 138.7 Cellular senescence : methods and protocols. New York, NY. 2018. ISBN 9781493989317.
- ↑ Passos, João F; Saretzki, Gabriele; Ahmed, Shaheda; Nelson, Glyn; Richter, Torsten; Peters, Heiko; Wappler, Ilka; Birket, Matthew J; Harold, Graham; Schaeuble, Karin; Birch-Machin, Mark A; Kirkwood, Thomas B. L; von Zglinicki, Thomas (1 May 2007). "Mitochondrial Dysfunction Accounts for the Stochastic Heterogeneity in Telomere-Dependent Senescence". PLoS Biology. 5 (5): e110. doi:10.1371/journal.pbio.0050110.
- ↑ Anisimov, Vladimir N.; Berstein, Lev M.; Egormin, Peter A.; Piskunova, Tatiana S.; Popovich, Irina G.; Zabezhinski, Mark A.; Tyndyk, Margarita L.; Yurova, Maria V.; Kovalenko, Irina G.; Poroshina, Tatiana E.; Semenchenko, Anna V. (September 2008). "Metformin slows down aging and extends life span of female SHR mice". Cell Cycle. 7 (17): 2769–2773. doi:10.4161/cc.7.17.6625.
- ↑ Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008). "Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor". PLOS Biology. 6 (12): 2853–2868. PMC 2592359
 . PMID 19053174. doi:10.1371/journal.pbio.0060301.
. PMID 19053174. doi:10.1371/journal.pbio.0060301.
- ↑ Coppé, Jean-Philippe; Patil, Christopher K; Rodier, Francis; Sun, Yu; Muñoz, Denise P; Goldstein, Joshua; Nelson, Peter S; Desprez, Pierre-Yves; Campisi, Judith (2 December 2008). "Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor". PLoS Biology. 6 (12): e301. doi:10.1371/journal.pbio.0060301.
- ↑ "Ageing research cluster in Cologne moves closer together". www.mpg.de. Retrieved 12 July 2021.
- ↑ "The Nobel Prize in Physiology or Medicine 2009". NobelPrize.org. Retrieved 16 June 2021.
- ↑ Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, et al. (August 2009). "Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity". Aging Cell. 8 (4): 460–72. PMC 3652804
 . PMID 19489743. doi:10.1111/j.1474-9726.2009.00493.x.
. PMID 19489743. doi:10.1111/j.1474-9726.2009.00493.x.
- ↑ "Harvard scientists reverse the aging process in mice – now for humans".
- ↑ Passos, João F; Nelson, Glyn; Wang, Chunfang; Richter, Torsten; Simillion, Cedric; Proctor, Carole J; Miwa, Satomi; Olijslagers, Sharon; Hallinan, Jennifer; Wipat, Anil; Saretzki, Gabriele; Rudolph, Karl Lenhard; Kirkwood, Tom B L; von Zglinicki, Thomas (January 2010). "Feedback between p21 and reactive oxygen production is necessary for cell senescence". Molecular Systems Biology. 6 (1): 347. doi:10.1038/msb.2010.5.
- ↑ 148.0 148.1 "Home". TrentAging2019. Retrieved 9 June 2021.
- ↑ "ENAS - home". www.agingstudies.eu. Retrieved 9 June 2021.
- ↑ "ENAS - home". www.agingstudies.eu. Retrieved 23 December 2021.
- ↑ Baker, Darren J.; Wijshake, Tobias; Tchkonia, Tamar; LeBrasseur, Nathan K.; Childs, Bennett G.; van de Sluis, Bart; Kirkland, James L.; van Deursen, Jan M. (November 2011). "Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders". Nature. 479 (7372): 232–236. doi:10.1038/nature10600.
- ↑ Baker, Darren J.; Childs, Bennett G.; Durik, Matej; Wijers, Melinde E.; Sieben, Cynthia J.; Zhong, Jian; A. Saltness, Rachel; Jeganathan, Karthik B.; Verzosa, Grace Casaclang; Pezeshki, Abdulmohammad; Khazaie, Khashayarsha; Miller, Jordan D.; van Deursen, Jan M. (February 2016). "Naturally occurring p16Ink4a-positive cells shorten healthy lifespan". Nature. 530 (7589): 184–189. doi:10.1038/nature16932.
- ↑ "IFOM: research on cellular aging opens new therapeutic perspectives for various diseases". ResearchItaly - IFOM: research on cellular aging opens new therapeutic perspectives for various diseases. Retrieved 12 June 2021.
- ↑ Nelson, Glyn; Wordsworth, James; Wang, Chunfang; Jurk, Diana; Lawless, Conor; Martin‐Ruiz, Carmen; von Zglinicki, Thomas (April 2012). "A senescent cell bystander effect: senescence‐induced senescence". Aging Cell. 11 (2): 345–349. doi:10.1111/j.1474-9726.2012.00795.x.
- ↑ Lopez-Otin, C; et al. (2013). "The hallmarks of aging". Cell. 153 (6): 1194–217. PMC 3836174
 . PMID 23746838. doi:10.1016/j.cell.2013.05.039.
. PMID 23746838. doi:10.1016/j.cell.2013.05.039.
- ↑ Mitchell, Sarah J; Martin-Montalvo, Alejandro; Mercken, Evi M; et al. (27 February 2014). "The SIRT1 Activator SRT1720 Extends Lifespan and Improves Health of Mice Fed a Standard Diet". Cell Reports. 6 (5): 836–843. PMC 4010117
 . PMID 24582957. doi:10.1016/j.celrep.2014.01.031.
. PMID 24582957. doi:10.1016/j.celrep.2014.01.031.
- ↑ Mercken, Evi M; Mitchell, Sarah J; Martin-Montalvo, Alejandro; et al. (16 June 2014). "SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass". Aging Cell. 13 (5): 787–796. PMC 4172519
 . PMID 24931715. doi:10.1111/acel.12220.
. PMID 24931715. doi:10.1111/acel.12220.
- ↑ Madeo, Frank; Zimmermann, Andreas; Maiuri, Maria Chiara; Kroemer, Guido (2 January 2015). "Essential role for autophagy in life span extension". Journal of Clinical Investigation. 125 (1): 85–93. doi:10.1172/JCI73946.
- ↑ Xu, Ming; Palmer, Allyson K; Ding, Husheng; Weivoda, Megan M; Pirtskhalava, Tamar; White, Thomas A; Sepe, Anna; Johnson, Kurt O; Stout, Michael B; Giorgadze, Nino; Jensen, Michael D; LeBrasseur, Nathan K; Tchkonia, Tamar; Kirkland, James L (19 December 2015). "Targeting senescent cells enhances adipogenesis and metabolic function in old age". eLife. 4: e12997. doi:10.7554/eLife.12997.
- ↑ Baker, Darren J.; Childs, Bennett G.; Durik, Matej; Wijers, Melinde E.; Sieben, Cynthia J.; Zhong, Jian; A. Saltness, Rachel; Jeganathan, Karthik B.; Verzosa, Grace Casaclang; Pezeshki, Abdulmohammad; Khazaie, Khashayarsha; Miller, Jordan D.; van Deursen, Jan M. (February 2016). "Naturally occurring p16Ink4a-positive cells shorten healthy lifespan". Nature. 530 (7589): 184–189. doi:10.1038/nature16932.
- ↑ Clara Correia‐Melo, Francisco DM Marques, Rhys Anderson, Graeme Hewitt, Rachael Hewitt, John Cole, Bernadette M Carroll, Satomi Miwa, Jodie Birch, Alina Merz, Michael D Rushton, Michelle Charles, Diana Jurk, Stephen WG Tait, Rafal Czapiewski, Laura Greaves, Glyn Nelson, Mohammad Bohlooly‐Y, Sergio Rodriguez‐Cuenca, Antonio Vidal‐Puig, Derek Mann, Gabriele Saretzki, Giovanni Quarato, Douglas R Green, Peter D Adams, Thomas von Zglinicki, Viktor I Korolchuk, João F Passos (2016). "Mitochondria are required for pro‐aging features of the senescent phenotype". The EMBO Journal. 35: 724–742. PMID 26848154. doi:10.15252/embj.201592862.
- ↑ "Mitochondria shown to trigger cell aging: Batteries of the cells shown to be essential for aging". ScienceDaily. Retrieved 7 June 2021.
- ↑ Writer, GEN Staff (2016-02-04). "Mitochondria Trigger Cell Aging Response". GEN - Genetic Engineering and Biotechnology News. Retrieved 7 June 2021.
- ↑ Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; DAmico, D.; Ropelle, E. R.; Lutolf, M. P.; Aebersold, R.; Schoonjans, K.; Menzies, K. J.; Auwerx, J. (17 June 2016). "NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice". Science. 352 (6292): 1436–1443. doi:10.1126/science.aaf2693.
- ↑ Yoshino, Jun; Mills, Kathryn F.; Yoon, Myeong Jin; Imai, Shin-ichiro (15 October 2011). "Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice". Cell Metabolism. 14 (4): 528–536. PMC 3204926
 . PMID 21982712. doi:10.1016/j.cmet.2011.08.014.
. PMID 21982712. doi:10.1016/j.cmet.2011.08.014.
- ↑ "What is NMN?". NMN.com. 5 May 2020.
- ↑ Strong, Randy; Miller, Richard A.; Antebi, Adam; Astle, Clinton M.; Bogue, Molly; Denzel, Martin S.; Fernandez, Elizabeth; Flurkey, Kevin; Hamilton, Karyn L.; Lamming, Dudley W.; Javors, Martin A.; Magalhães, João Pedro; Martinez, Paul Anthony; McCord, Joe M.; Miller, Benjamin F.; Müller, Michael; Nelson, James F.; Ndukum, Juliet; Rainger, G. Ed.; Richardson, Arlan; Sabatini, David M.; Salmon, Adam B.; Simpkins, James W.; Steegenga, Wilma T.; Nadon, Nancy L.; Harrison, David E. (October 2016). "Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α‐glucosidase inhibitor or a Nrf2‐inducer". Aging Cell. 15 (5): 872–884. doi:10.1111/acel.12496.
- ↑ Childs, B. G.; Baker, D. J.; Wijshake, T.; Conover, C. A.; Campisi, J.; van Deursen, J. M. (28 October 2016). "Senescent intimal foam cells are deleterious at all stages of atherosclerosis". Science. 354 (6311): 472–477. doi:10.1126/science.aaf6659.
- ↑ Correia‐Melo, Clara; Marques, Francisco DM; Anderson, Rhys; Hewitt, Graeme; Hewitt, Rachael; Cole, John; Carroll, Bernadette M; Miwa, Satomi; Birch, Jodie; Merz, Alina; Rushton, Michael D; Charles, Michelle; Jurk, Diana; Tait, Stephen WG; Czapiewski, Rafal; Greaves, Laura; Nelson, Glyn; Bohlooly‐Y, Mohammad; Rodriguez‐Cuenca, Sergio; Vidal‐Puig, Antonio; Mann, Derek; Saretzki, Gabriele; Quarato, Giovanni; Green, Douglas R; Adams, Peter D; Zglinicki, Thomas; Korolchuk, Viktor I; Passos, João F (April 2016). "Mitochondria are required for pro‐ageing features of the senescent phenotype". The EMBO Journal. 35 (7): 724–742. doi:10.15252/embj.201592862.
- ↑ Ben-Avraham, Danny; Govindaraju, Diddahally R.; Budagov, Temuri; Fradin, Delphine; Durda, Peter; et al. (2 June 2017). "The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature". Science Advances. 3 (6): e1602025. PMC 5473676
 . PMID 28630896. doi:10.1126/sciadv.1602025.
. PMID 28630896. doi:10.1126/sciadv.1602025.
- ↑ Jeon, Ok Hee; Kim, Chaekyu; Laberge, Remi-Martin; Demaria, Marco; Rathod, Sona; Vasserot, Alain P; Chung, Jae Wook; Kim, Do Hun; Poon, Yan; David, Nathaniel; Baker, Darren J; van Deursen, Jan M; Campisi, Judith; Elisseeff, Jennifer H (June 2017). "Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment". Nature Medicine. 23 (6): 775–781. doi:10.1038/nm.4324.
- ↑ Lopes-Paciencia, Stéphane; Saint-Germain, Emmanuelle; Rowell, Marie-Camille; Ruiz, Ana Fernández; Kalegari, Paloma; Ferbeyre, Gerardo (May 2019). "The senescence-associated secretory phenotype and its regulation". Cytokine. 117: 15–22. doi:10.1016/j.cyto.2019.01.013.
- ↑ Matthews, Stephen (21 December 2017). "Restricting calories can delay aging, says researcher". Mail Online. Retrieved 16 March 2021.
- ↑ "Bipolar Disorder Linked to Accelerated Epigenetic Aging". whatisepigenetics.com. Retrieved 3 January 2018.
- ↑ "Opening the door to treating ageing as a disease". The Lancet Diabetes & Endocrinology. 6 (8): 587. August 2018. doi:10.1016/S2213-8587(18)30214-6.
- ↑ Biogerontology Research Foundation (2 July 2018). "World Health Organization adds extension code for 'aging-related' via ICD-11". American Association for the Advancement of Science#EurekAlert!.
- ↑ Steve Hill (31 August 2018). "Getting Aging Classified as a Disease – Daria Khaltourina". Life Extension Advocacy Foundation.
- ↑ "Inching Towards the Regulatory Classification of Aging as a Disease". Fight Aging!. 3 September 2018.
- ↑ Oksana Andreiuk (12 September 2018). "Let's talk about the World Health Organisation recognising ageing as a disease risk factor, updating the ICD for the first time in 35 years.". Medium.
- ↑ Ruby, J Graham; Smith, Megan; Buffenstein, Rochelle. "Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age". eLife. 7. ISSN 2050-084X. doi:10.7554/eLife.31157.
- ↑ Beltrán-Sánchez, Hiram; Finch, Caleb. "Age is just a number". eLife. 7. ISSN 2050-084X. doi:10.7554/eLife.34427.
- ↑ Lopes-Paciencia, Stéphane; Saint-Germain, Emmanuelle; Rowell, Marie-Camille; Ruiz, Ana Fernández; Kalegari, Paloma; Ferbeyre, Gerardo (May 2019). "The senescence-associated secretory phenotype and its regulation". Cytokine. 117: 15–22. doi:10.1016/j.cyto.2019.01.013.
- ↑ "Scientists extend mice lifespan 12% by tweaking telomeres". Big Think. 2019-10-22. Retrieved 13 June 2021.
- ↑ "Scientists extend mice lifespan 12% by tweaking telomeres". Amazing Stories. 2019-10-24. Retrieved 13 June 2021.
- ↑ Nield, David. "Scientists Dramatically Extend The Lifespans of Mice in a Genius New Telomere Study". ScienceAlert. Retrieved 13 June 2021.
- ↑ "Identification of a key protein linked to aging". ScienceDaily. Retrieved 27 May 2021.
- ↑ Timmers, Paul R. H. J.; Wilson, James F.; Joshi, Peter K.; Deelen, Joris (December 2020). "Multivariate genomic scan implicates novel loci and haem metabolism in human aging". Nature Communications. 11 (1): 3570. doi:10.1038/s41467-020-17312-3.
- ↑ "Drug Reverses Age-Related Mental Decline Within Days In Mice". Drug Reverses Age-Related Mental Decline Within Days | UC San Francisco. Retrieved 8 July 2021.
- ↑ "Senescence research". Google Trends. Retrieved 16 April 2021.
- ↑ "Senescence research". books.google.com. Retrieved 16 April 2021.
- ↑ "Senescence". wikipediaviews.org. Retrieved 16 April 2021.