Difference between revisions of "Timeline of Moderna"
(→Sample questions) |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 32: | Line 32: | ||
| 2015 onwards || [[w:Unicorn (finance)|Unicorn]] status || Moderna reaches unicorn status, faster than companies like {{w|Uber}}, {{w|Dropbox}}, and {{w|Lyft}}.<ref name="Lavishly fund">{{cite web |title=Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine |url=https://www.statnews.com/2017/01/10/moderna-trouble-mrna/ |website=STAT |access-date=3 February 2022 |date=10 January 2017}}</ref><ref>{{cite web |title=Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine |url=https://www.statnews.com/2017/01/10/moderna-trouble-mrna/ |website=STAT |access-date=3 February 2022 |date=10 January 2017}}</ref> | | 2015 onwards || [[w:Unicorn (finance)|Unicorn]] status || Moderna reaches unicorn status, faster than companies like {{w|Uber}}, {{w|Dropbox}}, and {{w|Lyft}}.<ref name="Lavishly fund">{{cite web |title=Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine |url=https://www.statnews.com/2017/01/10/moderna-trouble-mrna/ |website=STAT |access-date=3 February 2022 |date=10 January 2017}}</ref><ref>{{cite web |title=Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine |url=https://www.statnews.com/2017/01/10/moderna-trouble-mrna/ |website=STAT |access-date=3 February 2022 |date=10 January 2017}}</ref> | ||
|- | |- | ||
| − | | 2018 onwards || {{w|Public company}} || Moderna becomes a {{w|public company}} via the largest biotech | + | | 2018 onwards || {{w|Public company}} || Moderna becomes a {{w|public company}} via the largest biotech {{w|initial public offering}} in history to date. |
|- | |- | ||
| 2020 onwards || {{w|COVID-19 vaccine}} || Amidst the {{w|COVID-19 pandemic}}, Moderna introduces its {{w|mRNA vaccine}}, and the company share price escalates after encouraging early results from clinical trial. | | 2020 onwards || {{w|COVID-19 vaccine}} || Amidst the {{w|COVID-19 pandemic}}, Moderna introduces its {{w|mRNA vaccine}}, and the company share price escalates after encouraging early results from clinical trial. | ||
|- | |- | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Full timeline== | ==Full timeline== | ||
| Line 103: | Line 83: | ||
| 2016 || January 11 || {{w|Cancer}} || || Partnership || Moderna announces a new collaboration with {{w|AstraZeneca}}, to discover, co-develop and co-commercialize messenger RNA (mRNA) therapeutic candidates for the treatment of a range of cancers. The collaboration is in addition to the agreement announced by the companies in 2013 to develop mRNA Therapeutics against cardiovascular, metabolic and renal diseases as well as some types of cancers.<ref>{{cite web |title=AstraZeneca and Moderna Therapeutics announce new collaboration to co-develop and co-commercialise immuno-oncology mRNA therapeutics™ |url=https://www.astrazeneca.com/media-centre/press-releases/2016/AstraZeneca-and-Moderna-Therapeutics-announce-new-collaboration-to-co-develop-and-co-commercialise-immuno-oncology-mRNA-therapeutics-11012016.html#! |website=www.astrazeneca.com |access-date=10 February 2022 |language=en}}</ref><ref name="Key Milestones"/> | | 2016 || January 11 || {{w|Cancer}} || || Partnership || Moderna announces a new collaboration with {{w|AstraZeneca}}, to discover, co-develop and co-commercialize messenger RNA (mRNA) therapeutic candidates for the treatment of a range of cancers. The collaboration is in addition to the agreement announced by the companies in 2013 to develop mRNA Therapeutics against cardiovascular, metabolic and renal diseases as well as some types of cancers.<ref>{{cite web |title=AstraZeneca and Moderna Therapeutics announce new collaboration to co-develop and co-commercialise immuno-oncology mRNA therapeutics™ |url=https://www.astrazeneca.com/media-centre/press-releases/2016/AstraZeneca-and-Moderna-Therapeutics-announce-new-collaboration-to-co-develop-and-co-commercialise-immuno-oncology-mRNA-therapeutics-11012016.html#! |website=www.astrazeneca.com |access-date=10 February 2022 |language=en}}</ref><ref name="Key Milestones"/> | ||
|- | |- | ||
| − | | 2016 || January 12 || HIV/AIDS || || Financial (funding) || The {{w|Bill & Melinda Gates Foundation}} awards Moderna an initial US$20 million grant to develop a novel, affordable combination of mRNA-based antibody therapeutics to help prevent HIV infection. <ref>{{cite web |last1=Writer |first1=GEN Staff |title=Moderna Wins Initial $20M Grant from Gates Foundation |url=https://www.genengnews.com/topics/omics/moderna-wins-initial-20m-grant-from-gates-foundation/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=1 February 2022 |date=12 January 2016}}</ref><ref>{{cite web |title=Gates Foundation Invests $20 Million to Advance HIV Care |url=https://philanthropynewsdigest.org/news/gates-foundation-invests-20-million-to-advance-hiv-care |website=philanthropynewsdigest.org |access-date=11 February 2022}}</ref> | + | | 2016 || January 12 || {{w|HIV/AIDS}} || || Financial (funding) || The {{w|Bill & Melinda Gates Foundation}} awards Moderna an initial US$20 million grant to develop a novel, affordable combination of mRNA-based antibody therapeutics to help prevent HIV infection. <ref>{{cite web |last1=Writer |first1=GEN Staff |title=Moderna Wins Initial $20M Grant from Gates Foundation |url=https://www.genengnews.com/topics/omics/moderna-wins-initial-20m-grant-from-gates-foundation/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=1 February 2022 |date=12 January 2016}}</ref><ref>{{cite web |title=Gates Foundation Invests $20 Million to Advance HIV Care |url=https://philanthropynewsdigest.org/news/gates-foundation-invests-20-million-to-advance-hiv-care |website=philanthropynewsdigest.org |access-date=11 February 2022}}</ref> |
|- | |- | ||
| 2016 || February 5 || || || Criticism || A ''[[w:Nature (magazine)|Nature]]'' editorial criticizes Moderna for not publishing any {{w|peer review}}ed papers on its technology, unlike most other emerging and established biotech companies, and compares its approach to that of the controversially failed {{w|Theranos}}.<ref>{{cite web |title=Research not fit to print |url=https://www.nature.com/articles/nbt.3488 |website=Nature Biotechnology |access-date=19 January 2022 |pages=115–115 |language=en |doi=10.1038/nbt.3488 |date=February 2016}}</ref> | | 2016 || February 5 || || || Criticism || A ''[[w:Nature (magazine)|Nature]]'' editorial criticizes Moderna for not publishing any {{w|peer review}}ed papers on its technology, unlike most other emerging and established biotech companies, and compares its approach to that of the controversially failed {{w|Theranos}}.<ref>{{cite web |title=Research not fit to print |url=https://www.nature.com/articles/nbt.3488 |website=Nature Biotechnology |access-date=19 January 2022 |pages=115–115 |language=en |doi=10.1038/nbt.3488 |date=February 2016}}</ref> | ||
| Line 113: | Line 93: | ||
| 2016 || June 6 || || || Partnership || Moderna announces collaboration with American pharmaceutical company {{w|Charles River Laboratories}}, which is expected to support Moderna to grow and advance its pipeline.<ref>{{cite web |title=Moderna Therapeutics and Charles River Laboratories Announce Strategic Collaboration to Scale Moderna’s Nonclinical Development Efforts for Novel mRNA Therapeutics {{!}} Charles River Laboratories International, Inc. |url=https://ir.criver.com/news-releases/news-release-details/moderna-therapeutics-and-charles-river-laboratories-announce |website=ir.criver.com |access-date=10 February 2022 |language=en}}</ref><ref>{{cite web |title=Charles River signs up to help Moderna with mRNA pipeline |url=https://www.fiercebiotech.com/cro/charles-river-signs-up-to-help-moderna-mrna-pipeline |website=FierceBiotech |access-date=13 February 2022 |language=en}}</ref> | | 2016 || June 6 || || || Partnership || Moderna announces collaboration with American pharmaceutical company {{w|Charles River Laboratories}}, which is expected to support Moderna to grow and advance its pipeline.<ref>{{cite web |title=Moderna Therapeutics and Charles River Laboratories Announce Strategic Collaboration to Scale Moderna’s Nonclinical Development Efforts for Novel mRNA Therapeutics {{!}} Charles River Laboratories International, Inc. |url=https://ir.criver.com/news-releases/news-release-details/moderna-therapeutics-and-charles-river-laboratories-announce |website=ir.criver.com |access-date=10 February 2022 |language=en}}</ref><ref>{{cite web |title=Charles River signs up to help Moderna with mRNA pipeline |url=https://www.fiercebiotech.com/cro/charles-river-signs-up-to-help-moderna-mrna-pipeline |website=FierceBiotech |access-date=13 February 2022 |language=en}}</ref> | ||
|- | |- | ||
| − | | 2016 || June 29 || Cancer || || Partnership || Moderna partners with {{w|Merck & Co.}} to develop and commercialize novel messenger RNA (mRNA)-based personalized cancer vaccines. The partnership aims to combine Merck’s immuno-oncology know-how with Moderna’s mRNA vaccine technology and GMP manufacturing capabilities.<ref>{{cite web |last1=Writer |first1=GEN Staff |title=Merck, Moderna to Develop mRNA-Based Personalized Cancer Vaccines |url=https://www.genengnews.com/topics/omics/merck-moderna-to-develop-mrna-based-personalized-cancer-vaccines/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=1 February 2022 |date=29 June 2016}}</ref><ref>{{cite web |title=Merck and Moderna Announce Strategic Collaboration to Advance Novel mRNA-Based Personalized Cancer Vaccines with KEYTRUDA® (pembrolizumab) for the Treatment of Multiple Types of Cancer |url=https://www.merck.com/news/merck-and-moderna-announce-strategic-collaboration-to-advance-novel-mrna-based-personalized-cancer-vaccines-with-keytruda-pembrolizumab-for-the-treatment-of-multiple-types-of-cancer/ |website=Merck.com |access-date=9 February 2022}}</ref><ref>{{cite web |title=Moderna Enters $200M+ Strategic Collaboration and License Agreement with Merck |url=https://www.goodwinlaw.com/news/2016/06/06_30_16_moderna-enters-200m-merck-strategic |website=www.goodwinlaw.com |access-date=9 February 2022 |language=en}}</ref> | + | | 2016 || June 29 || {{w|Cancer}} || || Partnership || Moderna partners with {{w|Merck & Co.}} to develop and commercialize novel messenger RNA (mRNA)-based personalized cancer vaccines. The partnership aims to combine Merck’s immuno-oncology know-how with Moderna’s mRNA vaccine technology and GMP manufacturing capabilities.<ref>{{cite web |last1=Writer |first1=GEN Staff |title=Merck, Moderna to Develop mRNA-Based Personalized Cancer Vaccines |url=https://www.genengnews.com/topics/omics/merck-moderna-to-develop-mrna-based-personalized-cancer-vaccines/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=1 February 2022 |date=29 June 2016}}</ref><ref>{{cite web |title=Merck and Moderna Announce Strategic Collaboration to Advance Novel mRNA-Based Personalized Cancer Vaccines with KEYTRUDA® (pembrolizumab) for the Treatment of Multiple Types of Cancer |url=https://www.merck.com/news/merck-and-moderna-announce-strategic-collaboration-to-advance-novel-mrna-based-personalized-cancer-vaccines-with-keytruda-pembrolizumab-for-the-treatment-of-multiple-types-of-cancer/ |website=Merck.com |access-date=9 February 2022}}</ref><ref>{{cite web |title=Moderna Enters $200M+ Strategic Collaboration and License Agreement with Merck |url=https://www.goodwinlaw.com/news/2016/06/06_30_16_moderna-enters-200m-merck-strategic |website=www.goodwinlaw.com |access-date=9 February 2022 |language=en}}</ref> |
|- | |- | ||
| 2016 || July 6 || {{w|Cystic fibrosis}} || || Partnership || Moderna partners with {{w|Boston}}-based biopharmaceutical company {{w|Vertex Pharmaceuticals}} to discover and develop messenger ribonucleic acid (mRNA) therapeutics for {{w|cystic fibrosis}}. Vertex agrees to pay Moderna up to US$275 million in development and regulatory milestones, as well as undisclosed, tiered royalties on future sales.<ref>{{cite web |title=UPDATED: Vertex, Moderna pair to create mRNA cystic fibrosis treatment |url=https://www.fiercebiotech.com/biotech/vertex-moderna-pair-to-create-mrna-cystic-fibrosis-treatment |website=FierceBiotech |access-date=1 February 2022 |language=en}}</ref> | | 2016 || July 6 || {{w|Cystic fibrosis}} || || Partnership || Moderna partners with {{w|Boston}}-based biopharmaceutical company {{w|Vertex Pharmaceuticals}} to discover and develop messenger ribonucleic acid (mRNA) therapeutics for {{w|cystic fibrosis}}. Vertex agrees to pay Moderna up to US$275 million in development and regulatory milestones, as well as undisclosed, tiered royalties on future sales.<ref>{{cite web |title=UPDATED: Vertex, Moderna pair to create mRNA cystic fibrosis treatment |url=https://www.fiercebiotech.com/biotech/vertex-moderna-pair-to-create-mrna-cystic-fibrosis-treatment |website=FierceBiotech |access-date=1 February 2022 |language=en}}</ref> | ||
| Line 119: | Line 99: | ||
| 2016 || July 26 || || AZD8601 || {{w|Clinical trial}} || Moderna announces the first trial from a collaboration with {{w|AstraZeneca}}, which was announced in 2013 to discover, develop and commercialize mRNA to treat serious cardiovascular, metabolic and renal diseases as well as cancer. The Clinical Trial Application (CTA) is to be conducted in Europe for a Phase 1 study of AZD8601, an investigational mRNA-based therapy that encodes for {{w|vascular endothelial growth factor}}-A (VEGF-A)<ref>{{cite web |title=Cash-rich Moderna to begin first AstraZeneca trial under collab deal |url=https://www.fiercebiotech.com/biotech/cash-rich-moderna-to-begin-first-astrazeneca-trial-under-collab-deal |website=FierceBiotech |access-date=1 February 2022 |language=en}}</ref><ref>{{cite web |title=Moderna Therapeutics Provides Mid-Year Corporate Update, Announces Continued Clinical Development Progress and Pipeline Acceleration |url=https://www.businesswire.com/news/home/20160726005747/en/Moderna-Therapeutics-Provides-Mid-Year-Corporate-Update-Announces-Continued-Clinical-Development-Progress-and-Pipeline-Acceleration |website=www.businesswire.com |access-date=2 February 2022 |language=en |date=26 July 2016}}</ref>, a factor that induces the development of tumor-associated blood vessels and provides the way for invasion of cancer cells.<ref>{{cite web |title=Vascular Endothelial Growth Factor A |url=https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/vascular-endothelial-growth-factor-a#:~:text=Vascular%20Endothelial%20Growth%20Factor%20A%20(VEGFA)%20is%20a%20factor%20that,for%20invasion%20of%20cancer%20cells. |website=sciencedirect.com |access-date=14 February 2022}}</ref> | | 2016 || July 26 || || AZD8601 || {{w|Clinical trial}} || Moderna announces the first trial from a collaboration with {{w|AstraZeneca}}, which was announced in 2013 to discover, develop and commercialize mRNA to treat serious cardiovascular, metabolic and renal diseases as well as cancer. The Clinical Trial Application (CTA) is to be conducted in Europe for a Phase 1 study of AZD8601, an investigational mRNA-based therapy that encodes for {{w|vascular endothelial growth factor}}-A (VEGF-A)<ref>{{cite web |title=Cash-rich Moderna to begin first AstraZeneca trial under collab deal |url=https://www.fiercebiotech.com/biotech/cash-rich-moderna-to-begin-first-astrazeneca-trial-under-collab-deal |website=FierceBiotech |access-date=1 February 2022 |language=en}}</ref><ref>{{cite web |title=Moderna Therapeutics Provides Mid-Year Corporate Update, Announces Continued Clinical Development Progress and Pipeline Acceleration |url=https://www.businesswire.com/news/home/20160726005747/en/Moderna-Therapeutics-Provides-Mid-Year-Corporate-Update-Announces-Continued-Clinical-Development-Progress-and-Pipeline-Acceleration |website=www.businesswire.com |access-date=2 February 2022 |language=en |date=26 July 2016}}</ref>, a factor that induces the development of tumor-associated blood vessels and provides the way for invasion of cancer cells.<ref>{{cite web |title=Vascular Endothelial Growth Factor A |url=https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/vascular-endothelial-growth-factor-a#:~:text=Vascular%20Endothelial%20Growth%20Factor%20A%20(VEGFA)%20is%20a%20factor%20that,for%20invasion%20of%20cancer%20cells. |website=sciencedirect.com |access-date=14 February 2022}}</ref> | ||
|- | |- | ||
| − | | 2016 || August 18 || | + | | 2016 || August 18 || {{w|Cancer}} || {{w|Pembrolizumab}}, intralesional mRNA 2752 || {{w|Clinical trial}} || Moderna launches a pilot study with the purpose to investigate the change in the immune microenvironment of high risk ductal carcinoma in situ (DCIS) after short term exposure to {{w|pembrolizumab}}, a humanized antibody used in cancer immunotherapy. A total of 48 participants would be enrolled.<ref>{{cite web |last1=Esserman |first1=Laura |title=Testing the Ability of Pembrolizumab and mRNA 2752 to Alter the Tumor Immune MicroEnvionment (TIME) of High Risk DCIS |url=https://clinicaltrials.gov/ct2/show/NCT02872025?spons=ModernaTX%2C+Inc.&draw=4&rank=44 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=15 January 2022}}</ref> |
|- | |- | ||
| 2016 || September 7 || {{w|Zika virus}} || mRNA-1893 || Financial (funding) || Moderna announces a funding award of US$8 million with the potential of up to $125 million from the {{w|Biomedical Advanced Research and Development Authority}} (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the {{w|United States Department of Health and Human Services}}, to accelerate development of a novel Zika mRNA vaccine.<ref>{{cite web |title=Moderna Announces Funding Award from BARDA for $8 Million with Potential of up to $125 Million to Accelerate Development of Zika Messenger RNA (mRNA) Vaccine |url=https://www.businesswire.com/news/home/20160907005586/en/Moderna-Announces-Funding-Award-from-BARDA-for-8-Million-with-Potential-of-up-to-125-Million-to-Accelerate-Development-of-Zika-Messenger-RNA-mRNA-Vaccine |website=www.businesswire.com |access-date=1 February 2022 |language=en |date=7 September 2016}}</ref><ref>{{cite web |last1=Writer |first1=GEN Staff |title=Moderna Wins Up to $125M from BARDA Toward Zika mRNA Vaccine |url=https://www.genengnews.com/topics/omics/moderna-wins-up-to-125m-from-barda-toward-zika-mrna-vaccine/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=2 February 2022 |date=7 September 2016}}</ref> | | 2016 || September 7 || {{w|Zika virus}} || mRNA-1893 || Financial (funding) || Moderna announces a funding award of US$8 million with the potential of up to $125 million from the {{w|Biomedical Advanced Research and Development Authority}} (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the {{w|United States Department of Health and Human Services}}, to accelerate development of a novel Zika mRNA vaccine.<ref>{{cite web |title=Moderna Announces Funding Award from BARDA for $8 Million with Potential of up to $125 Million to Accelerate Development of Zika Messenger RNA (mRNA) Vaccine |url=https://www.businesswire.com/news/home/20160907005586/en/Moderna-Announces-Funding-Award-from-BARDA-for-8-Million-with-Potential-of-up-to-125-Million-to-Accelerate-Development-of-Zika-Messenger-RNA-mRNA-Vaccine |website=www.businesswire.com |access-date=1 February 2022 |language=en |date=7 September 2016}}</ref><ref>{{cite web |last1=Writer |first1=GEN Staff |title=Moderna Wins Up to $125M from BARDA Toward Zika mRNA Vaccine |url=https://www.genengnews.com/topics/omics/moderna-wins-up-to-125m-from-barda-toward-zika-mrna-vaccine/ |website=GEN - Genetic Engineering and Biotechnology News |access-date=2 February 2022 |date=7 September 2016}}</ref> | ||
| Line 129: | Line 109: | ||
| 2016 || October 17 || || || Partnership || Moderna partners with {{w|Somerset, New Jersey}}-based multinational {{w|Catalent}} to use the latter company’s {{w|Madison, Wisconsin}}-based 100,000-square-foot facility for production of cancer-targeting messenger RNA vaccines. Catalent agrees to provide with its facility that boasts Catalent’s SMARTag and GPEx technology platforms, which work to optimize bioconjugates and cell lines, respectively.<ref>{{cite web |title=Moderna taps Catalent for clinical manufacturing |url=https://www.biopharmadive.com/news/moderna-catalent-manufacturing-plant/428431/ |website=BioPharma Dive |access-date=2 February 2022}}</ref> | | 2016 || October 17 || || || Partnership || Moderna partners with {{w|Somerset, New Jersey}}-based multinational {{w|Catalent}} to use the latter company’s {{w|Madison, Wisconsin}}-based 100,000-square-foot facility for production of cancer-targeting messenger RNA vaccines. Catalent agrees to provide with its facility that boasts Catalent’s SMARTag and GPEx technology platforms, which work to optimize bioconjugates and cell lines, respectively.<ref>{{cite web |title=Moderna taps Catalent for clinical manufacturing |url=https://www.biopharmadive.com/news/moderna-catalent-manufacturing-plant/428431/ |website=BioPharma Dive |access-date=2 February 2022}}</ref> | ||
|- | |- | ||
| − | | 2016 || December 21 || Zika virus || mRNA-1325 || Clinical trial || Moderna starts clinical trial with the purpose to evaluate Zika vaccine candidate mRNA-1325 in healthy adult subjects.<ref>{{cite web |title=A Phase 1, Randomized, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety and Immunogenicity of mRNA 1325 Zika Vaccine in Healthy Adults in a Non-endemic Zika Region |url=https://clinicaltrials.gov/ct2/show/NCT03014089?spons=ModernaTX%2C+Inc.&draw=5&rank=39 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=6 December 2019}}</ref> | + | | 2016 || December 21 || {{{w|Zika virus}} || mRNA-1325 || Clinical trial || Moderna starts clinical trial with the purpose to evaluate Zika vaccine candidate mRNA-1325 in healthy adult subjects.<ref>{{cite web |title=A Phase 1, Randomized, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety and Immunogenicity of mRNA 1325 Zika Vaccine in Healthy Adults in a Non-endemic Zika Region |url=https://clinicaltrials.gov/ct2/show/NCT03014089?spons=ModernaTX%2C+Inc.&draw=5&rank=39 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=6 December 2019}}</ref> |
|- | |- | ||
| 2017 || January 4 || || || Partnership || Moderna joins the Human Vaccines Project, a non-profit public-private partnership focused on decoding the human immune system to accelerate the development of vaccines and immunotherapies against major infectious diseases and cancer.<ref>{{cite web |title=Moderna Joins the Human Vaccines Project to Help Advance Fundamental Understanding of the Immune System |url=https://www.modernatx.com/sites/default/files/press_releases/moderna_human_vaccines_project_press_release_1.4.17-2.pdf |website=modernatx.com |access-date=16 February 2022}}</ref> | | 2017 || January 4 || || || Partnership || Moderna joins the Human Vaccines Project, a non-profit public-private partnership focused on decoding the human immune system to accelerate the development of vaccines and immunotherapies against major infectious diseases and cancer.<ref>{{cite web |title=Moderna Joins the Human Vaccines Project to Help Advance Fundamental Understanding of the Immune System |url=https://www.modernatx.com/sites/default/files/press_releases/moderna_human_vaccines_project_press_release_1.4.17-2.pdf |website=modernatx.com |access-date=16 February 2022}}</ref> | ||
|- | |- | ||
| − | | 2017 || January 9 || || || {{w|Drug pipeline}} || Moderna unveils new details about its pipeline and strategy at the J.P. Morgan Healthcare Conference in {{w|San Francisco}}. The company announces five vaccines in clinical trials. Two are for strains of influenza with potencial | + | | 2017 || January 9 || {{w|Influenza}}, {{w|Zika virus}}, {{w|Chikungunya virus}} || || {{w|Drug pipeline}} || Moderna unveils new details about its pipeline and strategy at the J.P. Morgan Healthcare Conference in {{w|San Francisco}}. The company announces five vaccines in clinical trials. Two are for strains of influenza with potencial to become pandemic. Another is for Zika virus, and a fourth, being developed with AstraZeneca, treats heart attacks. A fifth vaccine candidate, co-developed with {{w|Merck}}, is not revealed. Another vaccine, for Chikungunya virus, is reported to be ready to start trials.<ref>{{cite web |last1=Herper |first1=Matthew |title=Mysterious $5 Billion Biotech Firm Moderna Lays Out Drug Pipeline |url=https://www.forbes.com/sites/matthewherper/2017/01/09/mysterious-5-billion-biotech-firm-moderna-lays-out-drug-pipeline/?sh=79d7ae8a4eb5 |website=Forbes |access-date=2 February 2022 |language=en}}</ref><ref>{{cite web |title=Moderna Provides Pipeline and Full-Year Corporate Update |url=https://www.businesswire.com/news/home/20170109006270/en/Moderna-Provides-Pipeline-and-Full-Year-Corporate-Update |website=www.businesswire.com |access-date=9 February 2022 |language=en |date=9 January 2017}}</ref><ref>{{cite web |title=Moderna Provides Pipeline and Full-Year Corporate Update |url=https://www.biotech-finances.com/moderna-provides-pipeline-and-full-year-corporate-update/ |website=Biotech Finances |access-date=10 February 2022 |language=fr-FR |date=10 January 2017}}</ref> |
|- | |- | ||
| 2017 || April 27 || {{w|Influenza}} (H10N8) || mRNA-1440 || {{w|Clinical trial}} (result) || Moderna shows announces positive interim data from its ongoing Phase 1 study of mRNA-1440, an mRNA infectious disease vaccine against avian H10N8 influenza, demonstrating mRNA-1440 induced high levels of immunogenicity, and was safe and well tolerated.<ref>{{cite web |last1=Rockoff |first1=Jonathan D. |title=Startup Moderna Shows Promise in Vaccine Trial |url=https://www.wsj.com/articles/startup-moderna-shows-promise-in-vaccine-trial-1493308801 |website=Wall Street Journal |access-date=3 February 2022 |date=27 April 2017}}</ref><ref>{{cite web |title=Moderna Announces Positive Interim Phase 1 Clinical Data Demonstrating First mRNA Vaccine Candidate, mRNA-1440, Induces High Levels of Immunogenicity |url=https://www.modernatx.com/sites/default/files/press_releases/Moderna%20H10%20Data%20Press%20Release__FinalforBusinessWire_4.27.17_9am.pdf |website=modernatx.com |access-date=9 February 2022}}</ref><ref>{{cite web |title=Secretive Moderna reveals first-in-human data from mRNA vaccine tech |url=https://www.fiercepharma.com/vaccines/once-secretive-moderna-reveals-its-first-human-data-from-avian-flu-vaccine-trial |website=FiercePharma |access-date=2 February 2022 |language=en}}</ref> | | 2017 || April 27 || {{w|Influenza}} (H10N8) || mRNA-1440 || {{w|Clinical trial}} (result) || Moderna shows announces positive interim data from its ongoing Phase 1 study of mRNA-1440, an mRNA infectious disease vaccine against avian H10N8 influenza, demonstrating mRNA-1440 induced high levels of immunogenicity, and was safe and well tolerated.<ref>{{cite web |last1=Rockoff |first1=Jonathan D. |title=Startup Moderna Shows Promise in Vaccine Trial |url=https://www.wsj.com/articles/startup-moderna-shows-promise-in-vaccine-trial-1493308801 |website=Wall Street Journal |access-date=3 February 2022 |date=27 April 2017}}</ref><ref>{{cite web |title=Moderna Announces Positive Interim Phase 1 Clinical Data Demonstrating First mRNA Vaccine Candidate, mRNA-1440, Induces High Levels of Immunogenicity |url=https://www.modernatx.com/sites/default/files/press_releases/Moderna%20H10%20Data%20Press%20Release__FinalforBusinessWire_4.27.17_9am.pdf |website=modernatx.com |access-date=9 February 2022}}</ref><ref>{{cite web |title=Secretive Moderna reveals first-in-human data from mRNA vaccine tech |url=https://www.fiercepharma.com/vaccines/once-secretive-moderna-reveals-its-first-human-data-from-avian-flu-vaccine-trial |website=FiercePharma |access-date=2 February 2022 |language=en}}</ref> | ||
| Line 201: | Line 181: | ||
| 2019 || May 28 || || mRNA-3704 || {{w|Clinical trial}} || Moderna starts a clinical trial with the purpose to evaluate mRNA-3704 in patients with methylmalonic acidemia/aciduria (MMA) due to methylmalonyl-coenzyme A mutase (MUT) deficiency between 1 to 18 years of age with elevated plasma methylmalonic acid. As of February 2022, 0 participants would be enrolled.<ref>{{cite web |title=A Global, Phase 1/2, Open Label, Dose Escalation Study to Evaluate the Safety, Pharmacodynamics, and Pharmacokinetics of mRNA-3704 in Patients With Isolated Methylmalonic Acidemia Due to Methylmalonyl-CoA Mutase Deficiency |url=https://clinicaltrials.gov/ct2/show/NCT03810690?spons=ModernaTX%2C+Inc.&draw=4&rank=29 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=10 November 2020}}</ref> | | 2019 || May 28 || || mRNA-3704 || {{w|Clinical trial}} || Moderna starts a clinical trial with the purpose to evaluate mRNA-3704 in patients with methylmalonic acidemia/aciduria (MMA) due to methylmalonyl-coenzyme A mutase (MUT) deficiency between 1 to 18 years of age with elevated plasma methylmalonic acid. As of February 2022, 0 participants would be enrolled.<ref>{{cite web |title=A Global, Phase 1/2, Open Label, Dose Escalation Study to Evaluate the Safety, Pharmacodynamics, and Pharmacokinetics of mRNA-3704 in Patients With Isolated Methylmalonic Acidemia Due to Methylmalonyl-CoA Mutase Deficiency |url=https://clinicaltrials.gov/ct2/show/NCT03810690?spons=ModernaTX%2C+Inc.&draw=4&rank=29 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=10 November 2020}}</ref> | ||
|- | |- | ||
| − | | 2019 || June 26 || || {{w|mRNA-5671}}, {{w|pembrolizumab}} || {{w|Clinical trial}} || Moderna starts clinical trial with the purpose to evaluate Phase 2 dose of V941(mRNA-5671/V941) as a monotherapy and in combination with {{w|pembrolizumab}} infusion.<ref>{{cite web |title=A Phase 1, Open-Label, Multicenter Study to Assess the Safety and Tolerability of mRNA-5671/V941 as a Monotherapy and in Combination With Pembrolizumab in Participants With KRAS Mutant Advanced or Metastatic Non-Small Cell Lung Cancer, Colorectal Cancer or Pancreatic Adenocarcinoma |url=https://clinicaltrials.gov/ct2/show/NCT03948763 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=18 February 2022 |date=9 February 2022}}</ref> | + | | 2019 || June 26 || {{w|Cancer}} || {{w|mRNA-5671}}, {{w|pembrolizumab}} || {{w|Clinical trial}} || Moderna starts clinical trial with the purpose to evaluate Phase 2 dose of V941(mRNA-5671/V941) as a monotherapy and in combination with {{w|pembrolizumab}} infusion.<ref>{{cite web |title=A Phase 1, Open-Label, Multicenter Study to Assess the Safety and Tolerability of mRNA-5671/V941 as a Monotherapy and in Combination With Pembrolizumab in Participants With KRAS Mutant Advanced or Metastatic Non-Small Cell Lung Cancer, Colorectal Cancer or Pancreatic Adenocarcinoma |url=https://clinicaltrials.gov/ct2/show/NCT03948763 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=18 February 2022 |date=9 February 2022}}</ref> |
|- | |- | ||
| 2019 || July 18 || {{w|Cancer}} || mRNA-4157, {{w|pembrolizumab}} || {{w|Clinical Trial}} || Moderna starts a clinical trial with the purpose to assess whether postoperative adjuvant therapy with mRNA-4157 and pembrolizumab improves recurrence free survival (RFS) compared to pembrolizumab alone in participants with complete resection of cutaneous melanoma and a high risk of recurrence.<ref>{{cite web |title=A Phase 2 Randomized Study of Adjuvant Immunotherapy With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab Versus Pembrolizumab Alone After Complete Resection of High-Risk Melanoma (KEYNOTE- 942) |url=https://clinicaltrials.gov/ct2/show/NCT03897881?spons=ModernaTX%2C+Inc.&draw=4&rank=27 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=18 December 2021}}</ref> | | 2019 || July 18 || {{w|Cancer}} || mRNA-4157, {{w|pembrolizumab}} || {{w|Clinical Trial}} || Moderna starts a clinical trial with the purpose to assess whether postoperative adjuvant therapy with mRNA-4157 and pembrolizumab improves recurrence free survival (RFS) compared to pembrolizumab alone in participants with complete resection of cutaneous melanoma and a high risk of recurrence.<ref>{{cite web |title=A Phase 2 Randomized Study of Adjuvant Immunotherapy With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab Versus Pembrolizumab Alone After Complete Resection of High-Risk Melanoma (KEYNOTE- 942) |url=https://clinicaltrials.gov/ct2/show/NCT03897881?spons=ModernaTX%2C+Inc.&draw=4&rank=27 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=18 December 2021}}</ref> | ||
| Line 273: | Line 253: | ||
| 2021 || January 8 || {{w|COVID-19}} || [[w:Moderna COVID-19 vaccine|mRNA-1273]] || {{w|Authorization}} || British Prime Minister {{w|Boris Johnson}} approves the {{w|Moderna COVID-19 vaccine}} for use in the {{w|United Kingdom}}, being the third coronavirus vaccine to approved for use in the country. The {{w|Government of the United Kingdom}} pre-orders 17 million doses of the Moderna vaccine.<ref>{{cite web |title=Moderna becomes third Covid vaccine approved in the UK |url=https://www.bbc.com/news/health-55586410 |website=BBC News |access-date=9 February 2022 |date=8 January 2021}}</ref> | | 2021 || January 8 || {{w|COVID-19}} || [[w:Moderna COVID-19 vaccine|mRNA-1273]] || {{w|Authorization}} || British Prime Minister {{w|Boris Johnson}} approves the {{w|Moderna COVID-19 vaccine}} for use in the {{w|United Kingdom}}, being the third coronavirus vaccine to approved for use in the country. The {{w|Government of the United Kingdom}} pre-orders 17 million doses of the Moderna vaccine.<ref>{{cite web |title=Moderna becomes third Covid vaccine approved in the UK |url=https://www.bbc.com/news/health-55586410 |website=BBC News |access-date=9 February 2022 |date=8 January 2021}}</ref> | ||
|- | |- | ||
| − | | 2021 || January 11 || {{w|Influenza}}, {{w|HIV}}, {{w|Nipah virus}} || Flu vaccine (mRNA-1010, mRNA-1020, mRNA-1030), HIV vaccine (mRNA-1644 & mRNA-1574), Nipah virus (NiV) Vaccine (mRNA-1215) || {{w|Drug pipeline}} || Moderna announces development of three mRNA-based vaccines for seasonal influenza, {{w|HIV}} and {{w|Nipah virus}}, to be conducted in 2021.<ref name="sdsdf">{{cite web |title=Moderna is developing three new mRNA-based vaccines for seasonal flu, HIV and Nipah virus |url=https://techcrunch.com/2021/01/11/moderna-is-developing-three-new-mrna-based-vaccines-for-seasonal-flu-hiv-and-nipah-virus/ |website=TechCrunch |access-date=19 January 2022}}</ref><ref>{{cite web |title=Moderna Provides Business Update and Announces Three New Development Programs in Infectious Disease Vaccines |url=https://investors.modernatx.com/news/news-details/2021/Moderna-Provides-Business-Update-and-Announces-Three-New-Development-Programs-in-Infectious-Disease-Vaccines-01-11-2021/default.aspx |website=investors.modernatx.com |access-date=19 January 2022}}</ref> | + | | 2021 || January 11 || {{w|Influenza}}, {{w|HIV/AIDS}}, {{w|Nipah virus}} || Flu vaccine (mRNA-1010, mRNA-1020, mRNA-1030), HIV vaccine (mRNA-1644 & mRNA-1574), Nipah virus (NiV) Vaccine (mRNA-1215) || {{w|Drug pipeline}} || Moderna announces development of three mRNA-based vaccines for seasonal influenza, {{w|HIV}} and {{w|Nipah virus}}, to be conducted in 2021.<ref name="sdsdf">{{cite web |title=Moderna is developing three new mRNA-based vaccines for seasonal flu, HIV and Nipah virus |url=https://techcrunch.com/2021/01/11/moderna-is-developing-three-new-mrna-based-vaccines-for-seasonal-flu-hiv-and-nipah-virus/ |website=TechCrunch |access-date=19 January 2022}}</ref><ref>{{cite web |title=Moderna Provides Business Update and Announces Three New Development Programs in Infectious Disease Vaccines |url=https://investors.modernatx.com/news/news-details/2021/Moderna-Provides-Business-Update-and-Announces-Three-New-Development-Programs-in-Infectious-Disease-Vaccines-01-11-2021/default.aspx |website=investors.modernatx.com |access-date=19 January 2022}}</ref> |
|- | |- | ||
| 2021 || January 19 || {{w|COVID-19}} || [[w:Moderna COVID-19 vaccine|mRNA-1273]] || Authorization || Moderna announces approval of its [[w:Moderna COVID-19 vaccine|mRNA-1273]] {{w|COVID-19 vaccine}} by Swissmedic, the Swiss Agency for therapeutic products, for use in Switzerland. The authorization comes over a month after the federal government increased its confirmed order commitment of mRNA-1273 from 4.5 million to 7.5 million doses.<ref>{{cite web |title=Switzerland Authorizes Moderna’s mRNA COVID-19 Vaccine |url=https://pharmanewsintel.com/news/switzerland-authorizes-modernas-mrna-covid-19-vaccine |website=PharmaNewsIntelligence |access-date=12 February 2022 |date=19 January 2021}}</ref> | | 2021 || January 19 || {{w|COVID-19}} || [[w:Moderna COVID-19 vaccine|mRNA-1273]] || Authorization || Moderna announces approval of its [[w:Moderna COVID-19 vaccine|mRNA-1273]] {{w|COVID-19 vaccine}} by Swissmedic, the Swiss Agency for therapeutic products, for use in Switzerland. The authorization comes over a month after the federal government increased its confirmed order commitment of mRNA-1273 from 4.5 million to 7.5 million doses.<ref>{{cite web |title=Switzerland Authorizes Moderna’s mRNA COVID-19 Vaccine |url=https://pharmanewsintel.com/news/switzerland-authorizes-modernas-mrna-covid-19-vaccine |website=PharmaNewsIntelligence |access-date=12 February 2022 |date=19 January 2021}}</ref> | ||
| Line 305: | Line 285: | ||
| 2021 || June 3 || || || Supply || Moderna signs pact with {{w|UNICEF}} for {{w|Covid-19}} vaccine supply. UNICEF and its procurement partners, including the {{w|Pan American Health Organization}}, gains access to up to 34 million doses of Moderna’s COVID-19 vaccine, to be delivered in the fourth quarter 2021, with an option for up to 466 million doses in 2022.<ref>{{cite web |title=Moderna Partners with UNICEF, Lonza, Thermo Fisher for COVID-19 Vaccine |url=https://www.dcatvci.org/top-industry-news/moderna-partners-with-unicef-lonza-thermo-fisher-for-covid-vaccine/ |website=DCAT Value Chain Insights |access-date=4 February 2022 |date=3 June 2021}}</ref> | | 2021 || June 3 || || || Supply || Moderna signs pact with {{w|UNICEF}} for {{w|Covid-19}} vaccine supply. UNICEF and its procurement partners, including the {{w|Pan American Health Organization}}, gains access to up to 34 million doses of Moderna’s COVID-19 vaccine, to be delivered in the fourth quarter 2021, with an option for up to 466 million doses in 2022.<ref>{{cite web |title=Moderna Partners with UNICEF, Lonza, Thermo Fisher for COVID-19 Vaccine |url=https://www.dcatvci.org/top-industry-news/moderna-partners-with-unicef-lonza-thermo-fisher-for-covid-vaccine/ |website=DCAT Value Chain Insights |access-date=4 February 2022 |date=3 June 2021}}</ref> | ||
|- | |- | ||
| − | | 2021 || June 8 || Zika || mRNA-1893 || {{w|Clinical trial}} || Moderna launches a clinical trial with the purpose to evaluate 2 dose levels of messenger RNA (mRNA)-1893 Zika vaccine in comparison to a placebo control in healthy participants who are flavivirus-seronegative and in participants who are flavivirus-seropositive.<ref>{{cite web |title=A Phase 2, Randomized, Observer-Blind, Placebo-Controlled, Dose Confirmation Study to Evaluate the Safety, Tolerability, and Immunogenicity of Zika Vaccine mRNA-1893 in Adults Aged 18 Through 65 Years and Living in Endemic and Non-Endemic Flavivirus Areas |url=https://clinicaltrials.gov/ct2/show/NCT04917861?spons=ModernaTX%2C+Inc.&draw=3&rank=13 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=18 January 2022}}</ref> | + | | 2021 || June 8 || {{w|Zika virus}} || mRNA-1893 || {{w|Clinical trial}} || Moderna launches a clinical trial with the purpose to evaluate 2 dose levels of messenger RNA (mRNA)-1893 Zika vaccine in comparison to a placebo control in healthy participants who are flavivirus-seronegative and in participants who are flavivirus-seropositive.<ref>{{cite web |title=A Phase 2, Randomized, Observer-Blind, Placebo-Controlled, Dose Confirmation Study to Evaluate the Safety, Tolerability, and Immunogenicity of Zika Vaccine mRNA-1893 in Adults Aged 18 Through 65 Years and Living in Endemic and Non-Endemic Flavivirus Areas |url=https://clinicaltrials.gov/ct2/show/NCT04917861?spons=ModernaTX%2C+Inc.&draw=3&rank=13 |website=clinicaltrials.gov |publisher=clinicaltrials.gov |access-date=19 February 2022 |date=18 January 2022}}</ref> |
|- | |- | ||
| 2021 || July 6 || || || Team || Paul Burton becomes Moderna's {{w|chief marketing officer}}, joining the company after 16 years at {{w|Johnson & Johnson}}.<ref>{{cite web |title=Moderna appoints J&J veteran Paul Burton as new CMO |url=https://www.ardent-search.com/an-ardent-view/ibt29k4tuk2prl49443s3bt041yy6d |website=Ardent Executive Search {{!}} Life Science & Healthcare Recruitment |access-date=9 February 2022}}</ref> | | 2021 || July 6 || || || Team || Paul Burton becomes Moderna's {{w|chief marketing officer}}, joining the company after 16 years at {{w|Johnson & Johnson}}.<ref>{{cite web |title=Moderna appoints J&J veteran Paul Burton as new CMO |url=https://www.ardent-search.com/an-ardent-view/ibt29k4tuk2prl49443s3bt041yy6d |website=Ardent Executive Search {{!}} Life Science & Healthcare Recruitment |access-date=9 February 2022}}</ref> | ||
| Line 396: | Line 376: | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | == Numerical and visual data == | ||
| + | |||
| + | === Google Trends === | ||
| + | |||
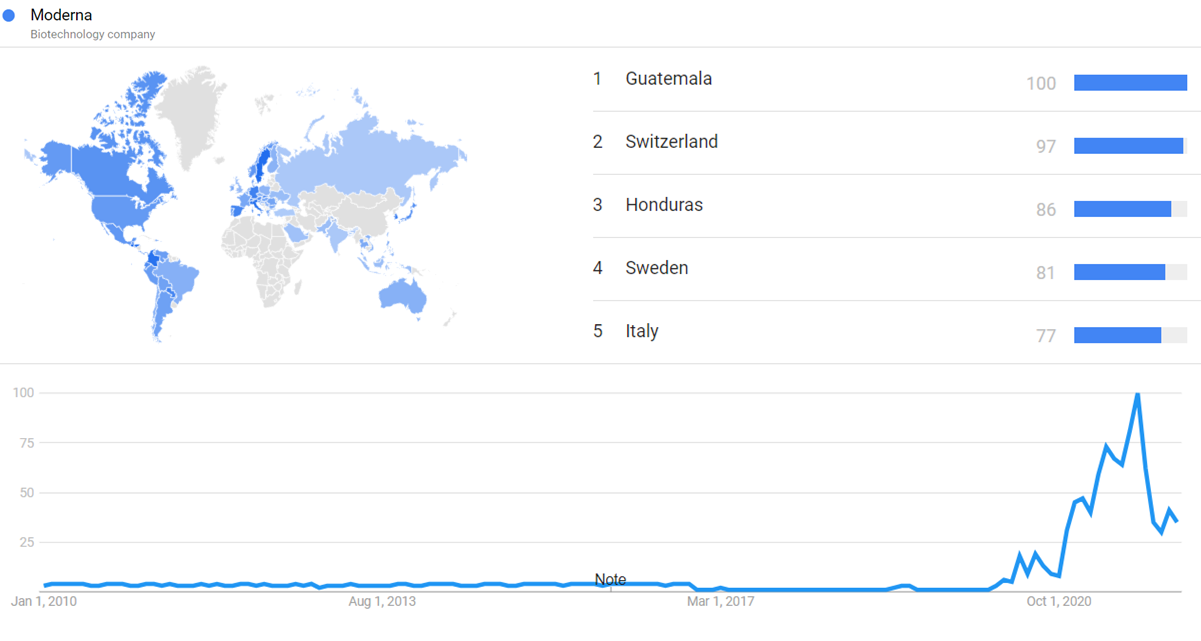
| + | The chart below shows {{w|Google Trends}} data for Moderna (Biotechnology Company), from January 2010 to January 2022, when the screenshot was taken. Interest is also ranked by country and displayed on world map. <ref>{{cite web |title=Moderna |url=https://trends.google.com/trends/explore?date=2010-01-01%202022-01-14&q=%2Fm%2F0w2ztxm |website=Google Trends |access-date=14 January 2022}}</ref> The first increase in early 2020 happens as COVID-19 starts spreading and Moderna announces its COVID-19 vaccine efforts. Further increases near the end of 2020 occur once Moderna succeeds in its human clinical trials of the COVID-19 vaccine. After that, interest in Moderna largely tracks the evolution of the COVID-19 pandemic. | ||
| + | |||
| + | [[File:Moderna gt.png|thumb|center|700px]] | ||
| + | |||
| + | === Google Finance === | ||
| + | |||
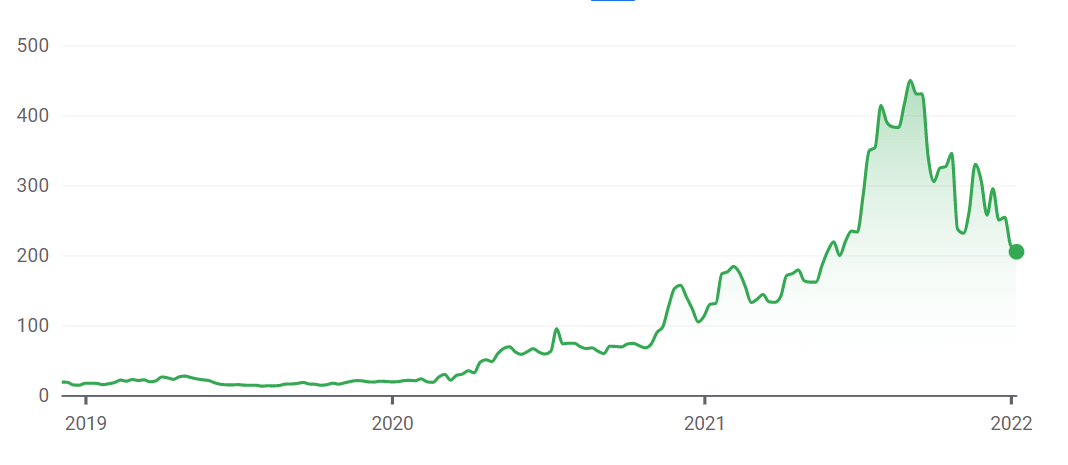
| + | The image below shows Moderna stock valuation in {{w|USD}} from {{w|IPO}} to January 14, 2022, when the screenshot was taken.<ref>{{cite web |title=Moderna |url=https://www.google.com/finance/quote/MRNA:NASDAQ?hl=en&window=MAX |website=www.google.com |access-date=14 January 2022 |language=en}}</ref> | ||
| + | |||
| + | [[File:Moderna gf.png|thumb|center|700px]] | ||
| + | |||
| + | === Wikipedia Views === | ||
| + | |||
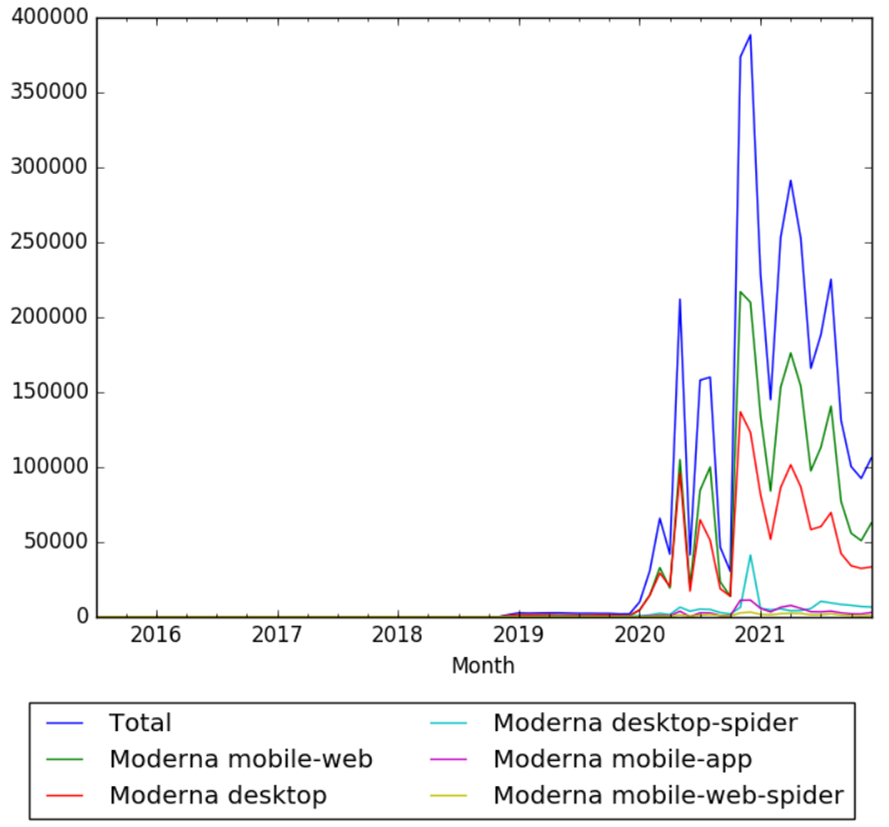
| + | The chart below shows pageviews of the English Wikipedia article {{w|Moderna}}, from July 2015 to December 2021.<ref>{{cite web |title=Moderna |url=https://wikipediaviews.org/displayviewsformultiplemonths.php?page=Moderna&allmonths=allmonths-api&language=en&drilldown=all |website=wikipediaviews.org |access-date=14 January 2022}}</ref> | ||
| + | |||
| + | [[File:Moderna wv.png|thumb|center|550px]] | ||
==Meta information on the timeline== | ==Meta information on the timeline== | ||
Latest revision as of 22:26, 24 March 2024
This is a timeline of Moderna, a Cambridge, Massachusetts-based pharmaceutical and biotechnology company that focuses on RNA therapeutics, primarily mRNA vaccines. An mRNA vaccine involves providing a person with just a set of instructions on how to build specific proteins that will trigger a body’s natural defenses.[1]
Contents
Sample questions
The following are some interesting questions that can be answered by reading this timeline:
- Which diseases and conditions are target of Moderna's drug pipeline?
- Sort the full timeline by "Disease/condition (when applicable)"
- For the names of their respective candidate drugs, sort the full timeline by "Product research name (when applicable)".
- What are the multiple clinical trials conducted by Moderna?
- Sort the full timeline by "Event type" and look for the group of rows with value "Clinical trial".
- You will mostly see trial launches, but also some sample or notable clinical trial results.
- What are some notable collaborations started between Moderna and other organizations?
- Sort the full timeline by "Event type" and look for the group of rows with value "Partnership".
- You will see collaborations with important companies such as Merck & Co. and AstraZeneca, as well as notable research institutes, such as Pasteur Institute.
- Who are some notable people having been related to Moderna over the years?
- Sort the full timeline by "Event type" and look for the group of rows with value "Team".
- What are some Moderna announcements on its drug pipeline?
- Sort the full timeline by "Event type" and look for the group of rows with value "Drug pipeline".
- You will see events describing updates on Moderna's pipeline and strategy.
- Other events are described under the following types: "Animal testing", "Business model", "Company launch", "Criticism", "Intellectual property", "Prelude", "Vaccine efficacy", and "Venture launch".
Big picture
| Time period | Development summary | More details |
|---|---|---|
| 2010–2012 | Early years | Early period right after the founding of the company. Moderna remains largely unknown with no external funding. |
| 2012 onwards | Jump to prominence | Moderna bursts onto the biotech scene in 2012, with its promising technology using messenger RNA (mRNA) to spur the production of human proteins within patient cells, effectively creating an in vivo factory for targeted therapies.[2] The company starts receiving funding rounds.[3] |
| 2015 onwards | Unicorn status | Moderna reaches unicorn status, faster than companies like Uber, Dropbox, and Lyft.[4][5] |
| 2018 onwards | Public company | Moderna becomes a public company via the largest biotech initial public offering in history to date. |
| 2020 onwards | COVID-19 vaccine | Amidst the COVID-19 pandemic, Moderna introduces its mRNA vaccine, and the company share price escalates after encouraging early results from clinical trial. |
Full timeline
| Year | Month and date (approximately) | Disease/condition (when applicable) | Product research name (when applicable) | Event type | Details |
|---|---|---|---|---|---|
| 2010 | Prelude | Canadian stem cell biologist Derrick Rossi, working at Harvard Medical School, builds upon Shinya Yamanaka's work on induced pluripotent stem cells and uses RNA to create embryonic stem cells from adult cells. This would eventually lead to the foundation of Moderna in the same year.[6] | |||
| 2010 | September | Company launch | Moderna is founded in Cambridge, Massachusetts, by Derrick Rossi, Robert S. Langer, Kenneth R. Chien, and venture capitalist Noubar Afeyan.[7] | ||
| 2012 | March | Recognition | A study highlights ModeRNA's program for RNA 3D structure prediction among a number of software projects deemed useful to plan a project, support the involvement of experts, and to promote higher quality and maintainability of programs.[8] | ||
| 2013 | March | Cardiovascular, metabolic, and renal diseases, cancer | Partnership | Moderna and AstraZeneca sign a five-year exclusive option agreement to discover, develop, and commercialize mRNA for treatments in the therapeutic areas of cardiovascular, metabolic, and renal diseases, and selected targets for cancer.[9][10][11][12] | |
| 2013 | May 22 | Team | John Reynders is hired by Moderna as Chief Information Officer, joining the company from AstraZeneca, where he served as Vice President of R&D Information.[13] | ||
| 2013 | October 2 | Financial (funding) | The United States Defense Advanced Research Projects Agency (DARPA) awards Moderna a US$24.6 million grant to develop messenger RNA therapeutics, Moderna's new treatment modality aimed to enable the in vivo production of therapeutic proteins. With the funding, DARPA expects a fast, cheap, and unusually adaptable method for fighting known contemporary and future pandemic threats.[14][15][16] | ||
| 2013 | October 17 | Infrastructure | Moderna announces the expansion of its headquarters to Technology Square in Cambridge, Massachusetts, with the addition of new space.[17][18][19] | ||
| 2013 | November 20 | Financial (funding) | Moderna raises US$110 million of equity financing.[20] | ||
| 2014 | January 13 | Rare diseases | Financial (funding) | AstraZeneca subsidiary Alexion Pharmaceuticals and Moderna announce an agreement for the discovery and development of messenger RNA Therapeutics to treat rare diseases. Alexion agrees to pay Moderna US$100 million to purchase 10 product options to develop and commercialize treatments for rare diseases with Moderna’s mRNA Therapeutics platform.[21][22][23] | |
| 2014 | March 6 | Team | Moderna appoints biotechnology investment banking veteran Lorence Kim as Chief Financial Officer.[24][25][26] | ||
| 2015 | January 5 | Financial (funding) | Moderna raises US$450 million in biggest biotechnology private round to date. Funders include Viking Global Investors LP, Invus, RA Capital Management, and Wellington Management Company, LLP, AstraZeneca and Alexion Pharmaceuticals.[27][28] | ||
| 2015 | January 13 | Financial (funding) | Merck agrees to pay US$50 million to Moderna for the rights to use its mRNA technology to develop as many as five vaccines and treatments against infectious diseases over the next three years. Merck also makes a US$50 million equity investment in Moderna. Adding up these payments, Moderna raises US$500 million in private investment in the month.[29][30] | ||
| 2015 | January | Financial (valuation) | Moderna reaches US$1 billion valuation, gaining unicorn status.[31] | ||
| 2015 | February 2 | Partnership | Moderna announces a long-term strategic research collaboration with Pasteur Institute for the discovery and development of drugs and vaccines using the company's mRNA platform. Moderna venture company Valera is appointed to manage the research collaboration for Moderna.[32] | ||
| 2015 | March 16 | Team | Tal Zaks becomes Moderna's chief medical officer, joining the company from Sanofi, where he was senior vice president and was responsible for all aspects of oncology drug discovery, development and commercialization.[33][34][35] | ||
| 2015 | October 13 | Team | Moderna Therapeutics' top scientist Joseph Bolen resigns from his positions as chief scientific officer and president of R&D.[2] | ||
| 2015 | October 22 | Cancer | Venture launch | Moderna launches a new personalized cancer vaccine venture, spinning out its messenger RNA tech into a separate entity dubbed Caperna.[36] | |
| 2015 | December | Influenza | VAL-506440 | Clinical trial | Moderna initiates a Phase 1 clinical trial to evaluate the safety and immunogenicity of H10N8 Antigen mRNA in healthy adult subjects, against seasonal influenza. A total of 201 participants would be enrolled.[37][38] |
| 2016 | January 11 | mRNA 1440, mRNA 1851 | Clinical trial | Moderna announces transition to a clinical stage company with its first Phase I study for mRNA 1440 currently underway in Europe. The company also announces having filed an investigational new drug application with the U.S. Food and Drug Administration for mRNA 1851, its second clinical program. Both mRNA 1440 and mRNA 1851 are infectious disease vaccines for undisclosed targets and indications.[39][40] | |
| 2016 | January 11 | Cancer | Partnership | Moderna announces a new collaboration with AstraZeneca, to discover, co-develop and co-commercialize messenger RNA (mRNA) therapeutic candidates for the treatment of a range of cancers. The collaboration is in addition to the agreement announced by the companies in 2013 to develop mRNA Therapeutics against cardiovascular, metabolic and renal diseases as well as some types of cancers.[41][42] | |
| 2016 | January 12 | HIV/AIDS | Financial (funding) | The Bill & Melinda Gates Foundation awards Moderna an initial US$20 million grant to develop a novel, affordable combination of mRNA-based antibody therapeutics to help prevent HIV infection. [43][44] | |
| 2016 | February 5 | Criticism | A Nature editorial criticizes Moderna for not publishing any peer reviewed papers on its technology, unlike most other emerging and established biotech companies, and compares its approach to that of the controversially failed Theranos.[45] | ||
| 2016 | May 9 | Team | Saqib Islam becomes Moderna's chief business officer, joining the company from Alexion Pharmaceuticals, where he was executive vice president, chief strategy and portfolio officer.[46][47][48] | ||
| 2016 | May 11 | Influenza | VAL-339851 | Clinical trial | Moderna starts Phase 1 clinical trial with the purpose to assess the safety, tolerability and immunogenicity of biological intervention VAL-339851 in healthy subjects. A total of 156 participants would be enrolled.[49] |
| 2016 | June 6 | Partnership | Moderna announces collaboration with American pharmaceutical company Charles River Laboratories, which is expected to support Moderna to grow and advance its pipeline.[50][51] | ||
| 2016 | June 29 | Cancer | Partnership | Moderna partners with Merck & Co. to develop and commercialize novel messenger RNA (mRNA)-based personalized cancer vaccines. The partnership aims to combine Merck’s immuno-oncology know-how with Moderna’s mRNA vaccine technology and GMP manufacturing capabilities.[52][53][54] | |
| 2016 | July 6 | Cystic fibrosis | Partnership | Moderna partners with Boston-based biopharmaceutical company Vertex Pharmaceuticals to discover and develop messenger ribonucleic acid (mRNA) therapeutics for cystic fibrosis. Vertex agrees to pay Moderna up to US$275 million in development and regulatory milestones, as well as undisclosed, tiered royalties on future sales.[55] | |
| 2016 | July 26 | AZD8601 | Clinical trial | Moderna announces the first trial from a collaboration with AstraZeneca, which was announced in 2013 to discover, develop and commercialize mRNA to treat serious cardiovascular, metabolic and renal diseases as well as cancer. The Clinical Trial Application (CTA) is to be conducted in Europe for a Phase 1 study of AZD8601, an investigational mRNA-based therapy that encodes for vascular endothelial growth factor-A (VEGF-A)[56][57], a factor that induces the development of tumor-associated blood vessels and provides the way for invasion of cancer cells.[58] | |
| 2016 | August 18 | Cancer | Pembrolizumab, intralesional mRNA 2752 | Clinical trial | Moderna launches a pilot study with the purpose to investigate the change in the immune microenvironment of high risk ductal carcinoma in situ (DCIS) after short term exposure to pembrolizumab, a humanized antibody used in cancer immunotherapy. A total of 48 participants would be enrolled.[59] |
| 2016 | September 7 | Zika virus | mRNA-1893 | Financial (funding) | Moderna announces a funding award of US$8 million with the potential of up to $125 million from the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the United States Department of Health and Human Services, to accelerate development of a novel Zika mRNA vaccine.[60][61] |
| 2016 | September 13 | Criticism | Stat publishes an article criticizing the Moderna's reputation for secrecy and the absence of scientific validation or independent peer review of its research, despite it then having the highest valuation of any U.S. private biotech company at over US$5 billion.[9] | ||
| 2016 | September 21 | Infrastructure | Moderna announces the lease of 200,000-square-foot facility for 15 years, for new clinical manufacturing facility in Norwood, Massachusetts.[62] The plant is expected to cost US$ 110 million.[63] DPS Group and Tria Architecture would be selected to provide architectural, engineering and project management services.[64] | ||
| 2016 | October 17 | Partnership | Moderna partners with Somerset, New Jersey-based multinational Catalent to use the latter company’s Madison, Wisconsin-based 100,000-square-foot facility for production of cancer-targeting messenger RNA vaccines. Catalent agrees to provide with its facility that boasts Catalent’s SMARTag and GPEx technology platforms, which work to optimize bioconjugates and cell lines, respectively.[65] | ||
| 2016 | December 21 | {Zika virus | mRNA-1325 | Clinical trial | Moderna starts clinical trial with the purpose to evaluate Zika vaccine candidate mRNA-1325 in healthy adult subjects.[66] |
| 2017 | January 4 | Partnership | Moderna joins the Human Vaccines Project, a non-profit public-private partnership focused on decoding the human immune system to accelerate the development of vaccines and immunotherapies against major infectious diseases and cancer.[67] | ||
| 2017 | January 9 | Influenza, Zika virus, Chikungunya virus | Drug pipeline | Moderna unveils new details about its pipeline and strategy at the J.P. Morgan Healthcare Conference in San Francisco. The company announces five vaccines in clinical trials. Two are for strains of influenza with potencial to become pandemic. Another is for Zika virus, and a fourth, being developed with AstraZeneca, treats heart attacks. A fifth vaccine candidate, co-developed with Merck, is not revealed. Another vaccine, for Chikungunya virus, is reported to be ready to start trials.[68][69][70] | |
| 2017 | April 27 | Influenza (H10N8) | mRNA-1440 | Clinical trial (result) | Moderna shows announces positive interim data from its ongoing Phase 1 study of mRNA-1440, an mRNA infectious disease vaccine against avian H10N8 influenza, demonstrating mRNA-1440 induced high levels of immunogenicity, and was safe and well tolerated.[71][72][73] |
| 2017 | May 16 | Intellectual property | Arbutus Biopharma Corporation CEO Mark Murray comments: We have seen no evidence of a Moderna delivery system that is free of our intellectual property. Not in their publications, their presentations, or in the examples in their patent filings, (...). In our view what they are reporting as theirs appears to be dominated by our intellectual property.[74] | ||
| 2017 | July 13 | Zika virus | mRNA-1893 | Animal testing | Moderna announces new data demonstrating that its Zika mRNA vaccine prevented Zika virus transmission from pregnant mice to their fetuses. The findings also demonstrated that Moderna’s Zika mRNA vaccine protected the placenta and fetus from Zika virus-induced injury.[75][76] |
| 2017 | July 27 | Partnership | Alexion Pharmaceuticals leaves Moderna, writing off a US$100 million partnership started in 2014. The pair had joined forces to use Moderna’s unproven technology to treat the rare and debilitating Crigler-Najjar syndrome, but their work would repeatedly run into safety issues, never making it into human trials.[77][78] | ||
| 2017 | August 3 | Team | Juan Andres is hired by Moderna as Senior Vice President of Late Stage Technical Development and Manufacturing, joining the company from Novartis, where he held the position of Global Head Technical Operations (manufacturing and supply).[79][80][81] | ||
| 2017 | August 9 | Relapsed/refractory solid tumor malignancies or lymphoma, ovarian cancer | mRNA-2416, Durvalumab | Clinical trial | Moderna starts clinical to evaluate escalating doses of mRNA-2416 alone and in combination with administered fixed doses of durvalumab in participants with relapsed/refractory solid tumor malignancies or lymphoma, as well as the objective response rate of mRNA-2416 alone or in combination with durvalumab in ovarian cancer.[82] |
| 2017 | August 14 | Cancer | mRNA-4157 | Clinical trial | Moderna launches clinical trial to evaluate cancer vaccine candidate mRNA-4157 alone in participants with resected solid tumors and in combination with pembrolizumab in participants with unresectable solid tumors.[83] |
| 2017 | August 15 | Chikungunya | mRNA-1388 | Clinical trial | Moderna starts a phase 1 clinical trial to evaluate chikungunya vaccine candidate mRNA-1388 (VAL-181388) in healthy subjects. This marks the fifth prophylactic vaccine to be pushed into the clinic by the company.[84][85][86] |
| 2017 | September 14 | Business model | Moderna announces decision to set aside its venture model (i.e., names and branding) in favor of "Therapeutic Area" R&D. The company also discloses three main therapeutic areas: infectious diseases, immuno-oncology and rare diseases.[87][88] | ||
| 2017 | October 27 | Cancer | mRNA-2416 | Clinical trial | Moderna announces launch of study with the purpose to assess the safety and tolerability of escalating doses of mRNA-2416 alone and in combination with administered fixed doses of durvalumab in participants with relapsed/refractory solid tumor malignancies or lymphoma, as well as the objective response rate of mRNA-2416 alone or in combination with durvalumab in ovarian cancer. The applicable dose of mRNA-2416 is to be injected directly into the participant's tumor (intratumoral) and the applicable dose of durvalumab is to be administered intravenously.[89][42] |
| 2017 | November 1 | Heart failure | AZD7970 | Partnership | Moderna partners with AstraZeneca to codevelop a relaxin mRNA treatment for heart failure. The candidate is designed to induce the body to produce relaxin, a hormone research groups see as a way to regulate the conditions that exacerbate heart failure.[90][91] |
| 2017 | November | Research | Moderna employees safely test mRNA technology in Sprague-Dawley rats and cynomolgus monkeys at the Montreal and Sherbrooke facilities of Charles River Laboratories. They find, among other things, that "mRNA is a labile biological molecule and therefore requires the use of protective delivery systems to effectively harness its potential," as the mRNA spread beyond the injection site and is found in the liver, spleen, bone marrow and heart.[92] | ||
| 2017 | November 13 | Cytomegalovirus | mRNA-1647 | Clinical trial | Moderna begins Phase I clinical trial of its vaccine candidate mRNA-1647 against cytomegalovirus, a member of the herpes virus family. The trial is scheduled to be completed on July 2020.[93][94] |
| 2017 | December 4 | Human parainfluenza, human metapneumovirus | mRNA-1653 | Clinical trial | Moderna begins dosing phase 1 subjects with mRNA-1653, a combination vaccine against human metapneumovirus (HMPV) and parainfluenza virus type 3 (PIV).[95][96][97] |
| 2018 | January 8 | Drug pipeline | Moderna announces pipeline update, including 19 mRNA drug candidates spanning infectious diseases, immuno-oncology, rare diseases and cardiovascular disorders, according to the company.[95][98] | ||
| 2018 | February 1 | Financial (funding) | Moderna raises US$500 million from private investors in Series G funding round, aimed at consolidating its pipeline of mRNA candidates and build out its manufacturing capabilities. Investors include Abu Dhabi Investment Authority, Julius Baer, the investment arm of the Singapore Economic Development Board and Sequoia Capital China. The series G leaves Moderna with US$1.4 billion in cash, with access to a further $250 million in grants.[99][100][101] | ||
| 2018 | March 7 | Team | Giuseppe Ciaramella, Moderna's CSO of the vaccines business, resigns from his position, leaving a long list of clinical programs behind.[102][103] | ||
| 2018 | March 20 | Methylmalonic acidemia, propionic acidemia | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate the signs and symptoms of patients with organic acidemias.[104][105] | |
| 2018 | May 3 | Cancer | mRNA-5671, other | Financial (funding) | Merck agrees to invest US$125 million in Moderna under a new agreement that expands the firms' 2016 partnership to develop messenger RNA (mRNA) cancer vaccines. The collaboration expansion includes partnering on cancer vaccine mRNA-5671, and other shared antigen mRNA cancer vaccines.[106][107][108][109] |
| 2018 | July 17 | Infrastructure | Moderna opens a $110 million 200,000 square foot facility in Norwood, Massachusetts for manufacturing, preclinical and clinical work.[110][111][112][113][42] | ||
| 2018 | October 25 | Recognition | The annual Science Careers Top Employers Survey recognizes Moderna as one of the global biopharmaceutical industry’s top employers in a fourth consecutive year. The annual employee survey evaluates companies in biotechnology, biopharmaceutical, pharmaceutical and other related industries in categories such as leadership in innovation, alignment between work culture and values and commitment to social responsibility. "Moderna Named a Top Employer by Science for the Fourth Consecutive Year"[114] | ||
| 2018 | November 27 | mRNA-2752 | Clinical trial | Moderna starts a clinical trial with the purpose to assess the safety and tolerability of escalating intratumoral doses of mRNA-2752 in participants with relapsed/refractory solid tumor malignancies or lymphoma. Over 260 participants would be enrolled.[115] | |
| 2018 | December 6 | Financial (Initial public offering) | Moderna becomes a public company via the largest biotech initial public offering in history to date, raising US$621 million (27 million shares at $23 per share).[116][117][118][119] "Moderna: 5 things to know about the largest biotech to IPO"[120] | ||
| 2019 | January 8 | Drug pipeline | Moderna announces updates to several of its immuno-oncology and rare disease programs, including 21 mRNA development candidates in its pipeline, with 11 programs in clinical development.[121] | ||
| 2019 | January 9 | Drug pipeline | At the JP Morgan Healthcare conference, Moderna CEO Stéphane Bancel gives an update on the company R&D plans, announcing from the data generated to date from its messenger RNA program three clear readouts: the mRNAs are all well-tolerated, express the designed protein, and those proteins are active in humans. At this time, Moderna has 21 development programs spanning prophylactic vaccines, cancer vaccines, immuno-oncology, and others, with 11 already in trials.[122] | ||
| 2019 | January 22 | Chikungunya | mRNA-1944 | Clinical trial | Moderna starts a Phase 1 first in human single-center randomized placebo controlled dose escalation study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of chikungunya virus antibody mRNA-1944 in healthy adult subjects.[123] On February 5 the company announces the first subject. This is the first development candidate from Moderna's systemic therapeutics modalities to start human dosing. The drug is administered via intravenous infusion in healthy adults.[124] |
| 2019 | February 12 | Human metapneumovirus, human parainfluenza | mRNA-1653 | Clinical trial (result) | Moderna announces positive interim phase 1 data for first combination vaccine against the human metapneumovirus and human parainfluenza. Data show vaccination with mRNA-1653 boosted serum neutralization titers against hMPV and PIV3 at all dose levels tested and was generally well tolerated.[125][126] |
| 2019 | February 20 | Vascular endothelial growth factor A (VEGF-A) | Clinical trial (result) | Moderna announces the publication of a Phase 1a/b study showing the potential of mRNA encoding for vascular endothelial growth factor A (VEGF-A) as a regenerative therapeutic, an approach that aims to stimulate the growth of new blood vessels, also known as angiogenesis, to improve blood flow in tissues where it is otherwise restricted.[127] | |
| 2019 | April 2 | Recognition | Moderna's clinical development manufacturing facility in Norwood, Massachusetts, wins the 2019 Facility of the Future Award, a nomination by the International Society of Pharmaceutical Engineers, whose awards program aims to recognize innovation and creativity in the pharmaceutical and biotechnology manufacturing industries.[128] | ||
| 2019 | May 10 | Influenza | Clinical trial (result) | Moderna announces the publication of positive results from two Phase 1 clinical studies showing that mRNA vaccines against influenza A virus subtype H10N8and and H7N9 were well-tolerated and elicited robust immune responses. In the H10N8 trial, 201 healthy volunteers 17 to 64 years of age received two doses of the vaccine or placebo three weeks apart, either intramuscularly or intradermally at two different doses. In the H7N9 trial, 156 healthy volunteers between the age of 17 and 49 years received two doses of vaccine or placebo three weeks apart at one of three doses, 10 µg, 25 µg or 50 µg. The results support the potential of mRNA-based vaccines to quickly and effectively address pandemic influenza strains.[129] | |
| 2019 | May 23 | Chikungunya | Clinical trial (result) | Moderna announces the publication of preclinical data showing that its encoding antibody against chikungunya virus is 100% protective against infection in animals. The developmental vaccine candidate mRNA-1944 encodes a human monoclonal antibody against the virus delivered in a proprietary lipid nanoparticle, protecting from infection by the virus in the living body of a plant or animal.[130] | |
| 2019 | May | Methylmalonic acidemia | mRNA-3704 | Clinical trial | Moderna starts a clinical trial of its candidate mRNA-3704 for treatment of patients with methylmalonic acidemia. This trial would not be completed.[131] |
| 2019 | May 28 | mRNA-3704 | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate mRNA-3704 in patients with methylmalonic acidemia/aciduria (MMA) due to methylmalonyl-coenzyme A mutase (MUT) deficiency between 1 to 18 years of age with elevated plasma methylmalonic acid. As of February 2022, 0 participants would be enrolled.[132] | |
| 2019 | June 26 | Cancer | mRNA-5671, pembrolizumab | Clinical trial | Moderna starts clinical trial with the purpose to evaluate Phase 2 dose of V941(mRNA-5671/V941) as a monotherapy and in combination with pembrolizumab infusion.[133] |
| 2019 | July 18 | Cancer | mRNA-4157, pembrolizumab | Clinical Trial | Moderna starts a clinical trial with the purpose to assess whether postoperative adjuvant therapy with mRNA-4157 and pembrolizumab improves recurrence free survival (RFS) compared to pembrolizumab alone in participants with complete resection of cutaneous melanoma and a high risk of recurrence.[134] |
| 2019 | July 30 | Clinical trial | mRNA-1893 | Clinical Trial | Moderna starts clinical trial to evaluate mRNA-1893 Zika vaccines in flavivirus seronegative and flavivirus seropositive participants.[135] |
| 2019 | August 19 | Zika virus | mRNA-1893 | Drug permission | Moderna is granted Fast Track designation by the U.S. Food and Drug Administration, for its investigational Zika vaccine (mRNA-1893) at the time being evaluated in a Phase 1 study for the prevention of Zika virus infection in healthy adults.[136] |
| 2019 | September 4 | Team | Tracey Franklin is appointed as Moderna's Chief Human Resources Officer, joining the company from Merck & Co., where she worked as Chief Talent and Strategy Officer.[137] | ||
| 2019 | September 12 | Cytomegalovirus | mRNA-1647 | Clinical trial (result) | Moderna announces positive data from the 3-month interim analysis of safety and immunogenicity of the Phase 1 clinical study of its investigational vaccine mRNA-1647, a 2-antigen vaccine designed to protect against cytomegalovirus infection. The drug combines 6 mRNAs in a proprietary lipid nanoparticle in a single vial and encodes for 2 immuno-dominant proteins of CMV.[138] |
| 2019 | September 12 | Chikungunya | mRNA-1944 | Clinical trial (result) | Moderna announces positive Phase 1 results for the first systemic mRNA therapeutic against chikungunya infection encoding a secreted protein (mRNA-1944), which was successfully encoded for functional antibody (CHKV-24) in humans at all dose levels tested (0.1, 0.3 and 0.6 mg/kg). Antibody level was achieved within hours, and projected to be maintained for at least 16 weeks at the middle and high doses.[139] |
| 2019 | October 2 | Infrastructure | Moderna announces expansion of its manufacturing site in Norwood, Massachusetts, with the addition of an 85,000 square-foot technical development center aimed to support Moderna's mRNA pipeline.[140][141] | ||
| 2019 | October 22 | Propionic acidemia | mRNA-3927 | Drug permission | The U.S. Food and Drug Administration grants Fast Track designation for Moderna's mRNA-3927, the company's investigational mRNA therapeutic for propionic acidemia.[142] Fast Track is one of five FDA approaches to make new drugs available as rapidly as possible.[143] |
| 2019 | October 24 | Recognition | Moderna is named Top Employer by Science and Science Careers' 2019 annual Top Employers Survey, which polls employees in the biotechnology, biopharmaceutical, pharmaceutical, and related industries to determine the 20 best employers in these industries as well as their driving characteristics.[144] | ||
| 2019 | November 4 | mRNA-1653 | Clinical trial | Moderna starts a clinical trial with the purpose to assess the safety and immunogenicity of 2 dose levels of mRNA-1653, a combined human metapneumovirus and human parainfluenza virus type 3 vaccine, in healthy adults (18 to 49 years of age) and 2 dose levels in children (12 to 59 months of age) with serologic evidence of prior exposure. Over 50 participants would be enrolled.[145] | |
| 2020 | January 9 | mRNA-1647 | Clinical trial | Moderna starts clinical study with the purpose to assess the safety and immunogenicity of 3 dose levels of mRNA-1647 cytomegalovirus vaccine in CMV-seronegative and CMV-seropositive healthy adults 18-40 years of age. Over 300 participants would be enrolled.[146] | |
| 2020 | January 23 | COVID-19 | mRNA-1273 | Financial (funding) | Moderna announces a new collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI), which agrees to fund the company to develop an mRNA vaccine against the novel coronavirus COVID-19.[147][148] |
| 2020 | February 10 | Methylmalonic Acidemia | mRNA-3704 | Clinical trial | Moderna announces first patient enrolled in the Phase I/II trial of mRNA-3704 in methylmalonic acidemia (MMA) patients, in what is the company’s first rare disease program to enter clinical trials. The drug is administered through intravenous infusion in patients with isolated MMA due to mitochondrial enzyme methylmalonic-CoA mutase (MUT) deficiency.[149] "Moderna enrols first patient in Phase I/II trial of mRNA-3704"[150] |
| 2020 | March 16 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts clinical trial designed to assess the safety, reactogenicity and immunogenicity of mRNA-1273, Moderna's COVID-19 vaccine. 120 participants would be enrolled.[151] |
| 2020 | May 1 | COVID-19 | Partnership | Moderna partners with Swiss firm Lonza to manufacture up to 1 billion doses a year of the potential COVID-19 vaccine.[152] | |
| 2020 | May 29 | COVID-19 | mRNA-1273 | Clinical trial | Moderna announces having dosed the first patients in Phase 2 Study of COVID-19 vaccine mRNA-1273. The study aims to evaluate the safety, reactogenicity and immunogenicity of two vaccinations of mRNA-1273 given 28 days apart.[153][154][155] |
| 2020 | June 4 | Team | David Meline is hired as Moderna's chief financial officer, joining the company from industrial conglomerate 3M where he also filled a CFO position.[156] | ||
| 2020 | June 25 | COVID-19 | mRNA-1273 | Partnership | Moderna partners with Catalent with the purpose to conduct large-scale, commercial fill-finish manufacturing of Moderna’s mRNA-based COVID-19 vaccine candidate (mRNA-1273) at Catalent’s biologics facility in Bloomington, Indiana.[157][158] |
| 2020 | July 14 | COVID-19 | mRNA-1273 | Research | The Moderna COVID-19 vaccine candidate is shown to be immunogenic in a Phase I trial involving 45 volunteers aged 18–55 years.[159] |
| 2020 | July 26 | mRNA-1273 | Financial (funding) | Moderna receives an additional US$472 million from the U.S. Biomedical Advanced Research and Development Authority to support development of its novel COVID-19 vaccine. The additional funding aims to support Moderna's late-stage clinical development including the expanded Phase 3 study of vaccine candidate mRNA-1273.[160] | |
| 2020 | July 27 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts clinical trial with the purpose to evaluate mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19.[161] |
| 2020 | August 11 | COVID-19 | mRNA-1273 | Supply | The United States Department of Health and Human Services and the United States Department of Defense announce an agreement with Moderna to manufacture and deliver 100 million doses of the company's COVID-19 vaccine candidate. The vaccine doses would be owned by the federal government.[162][163] |
| 2020 | September 16 | Cystic fibrosis | Partnership | Moderna announces a three-year strategic research collaboration and licensing agreement with Vertex Pharmaceuticals, aimed at the discovery and development of lipid nanoparticles and mRNAs for the delivery of gene-editing therapies for the treatment of cystic fibrosis. The initial focus of the partnership focuses on the discovery and optimization of novel LNPs and mRNAs that can deliver gene-editing therapies to cells in the lungs, enabling functional cystic fibrosis transmembrane conductance regulator protein to be produced.[164][165][166] | |
| 2020 | September 30 | mRNA-1345 | Clinical trial | Moderna starts a clinical trial aimed to evaluate the tolerability and reactogenicity of a single injection of up to 5 dose levels of mRNA-1345 in younger adults, women of child-bearing potential, and older adults including Japanese older adults; of 3 injections of the middle dose level of mRNA-1345 given 56 days apart in younger adults; of a booster injection of mRNA-1345 given approximately 12 months after the primary injection in older adults; and of 3 injections of 1 of 2 dose levels of mRNA-1345 given 56 days apart in children who are RSV-seropositive.[167] | |
| 2020 | October 29 | COVID-19 | mRNA-1273 | Supply | Takeda Pharmaceutical Company announced the import and distribution of 50 million doses of Moderna’s COVID-19 vaccine candidate, mRNA-1273, starting in the first half of 2021. The supply is arranged through a partnership with Moderna and the Government of Japan.[168] |
| 2020 | November 17 | Pulmonary arterial hypertension | Partnership | Moderna partners with Italian global pharmaceutical company Chiesi Farmaceutici with the purpose to discover and develop messenger RNA (mRNA) therapeutics to treat pulmonary arterial hypertension (PAH).[169] | |
| 2020 | November 30 | COVID-19 | mRNA-1273 | Clinical trial (result) | Moderna announces having found a 94.1 percent vaccine efficiency against the coronavirus in a second primary analysis of its COVID-19 vaccine candidate, mRNA-1273.[170][171] |
| 2020 | November 30 | COVID-19 | mRNA-1273 | Supply | Moderna and UKsigns a deal with the Government of the United Kingdom with the purpose to supply an additional two million doses of its Covid-19 vaccine candidate, from March 2021.[172] |
| 2020 | December 9 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts clinical trial to primarily evaluate the safety and reactogenicity of a single dose level of mRNA-1273 vaccine administered in 2 doses 28 days apart to an adolescent population.[173][174][175] |
| 2020 | December 18 | COVID-19 | mRNA-1273 | Authorization | Moderna's COVID-19 vaccine mRNA-1273 becomes the second to earn authorization by the U.S. Food and Drug Administration, one week after the emergency use authorization was granted to the Pfizer–BioNTech COVID-19 vaccine.[176][177][178] |
| 2020 | December 24 | COVID-19 | mRNA-1273 | Supply | Moderna receives authorization for its COVID-19 vaccine from Health Canada for the immunization of individuals aged 18 years and above in the country.[179] |
| 2021 | January 4 | COVID-19 | mRNA-1273 | Supply | The Ministry of Health of Israel authorizes the import of Moderna COVID-19 vaccine in the country, having secured 6 million doses.[180] |
| 2021 | January 4 | COVID-19 | mRNA-1273 | Supply | Moderna provides a supply update for its COVID-19 vaccine mRNA-1273, increasing its base-case global production estimate from 500 to 600 million doses for 2021. The company announces continued investment and addition of staff to build up to potentially 1 billion doses for 2021.[181][182] |
| 2021 | January 6 | COVID-19 | mRNA-1273 | Authorization | The European Commission grants conditional marketing authorization (CMA) for Moderna COVID-19 vaccine, following recommendations from the European Medicines Agency to use the candidate for active immunization to prevent the coronavirus in individuals 18 years of age and older. The conditional grant allows health officials to roll out the vaccine across the EU throughout 2021, including to its 27 member states. "[183][184] |
| 2021 | January 8 | COVID-19 | mRNA-1273 | Authorization | British Prime Minister Boris Johnson approves the Moderna COVID-19 vaccine for use in the United Kingdom, being the third coronavirus vaccine to approved for use in the country. The Government of the United Kingdom pre-orders 17 million doses of the Moderna vaccine.[185] |
| 2021 | January 11 | Influenza, HIV/AIDS, Nipah virus | Flu vaccine (mRNA-1010, mRNA-1020, mRNA-1030), HIV vaccine (mRNA-1644 & mRNA-1574), Nipah virus (NiV) Vaccine (mRNA-1215) | Drug pipeline | Moderna announces development of three mRNA-based vaccines for seasonal influenza, HIV and Nipah virus, to be conducted in 2021.[1][186] |
| 2021 | January 19 | COVID-19 | mRNA-1273 | Authorization | Moderna announces approval of its mRNA-1273 COVID-19 vaccine by Swissmedic, the Swiss Agency for therapeutic products, for use in Switzerland. The authorization comes over a month after the federal government increased its confirmed order commitment of mRNA-1273 from 4.5 million to 7.5 million doses.[187] |
| 2021 | January 19 | Team | Corinne Le Goff becomes Moderna's chief commercial officer, joining the company from previous roles in Amgen, Roche, and other large-cap pharma companies.[188][189][190] | ||
| 2021 | January 21 | COVID-19 | mRNA-1273 | Clinical trial | Moderna announces having dosed the first participant in the Phase 1/2 study of vaccine candidate against COVID-19 (mRNA-1273 or TAK-919) in Japan, led by Takeda Pharmaceutical Company, which intends to enroll 200 participants aged 20 years and above in the country. The study aims to evaluate the safety and immunogenicity of two vaccinations of mRNA-1273 given 28 days apart.[191][192] |
| 2021 | March 5 | Team | Moderna appoints Jonathan Hoggatt as director of hematology, joining the company from his position as Harvard Stem Cell Researcher.[193] | ||
| 2021 | March 8 | COVID-19 | mRNA-1273 | Partnership | Moderna partners with health care company Baxter International to produce up to 90 million doses of the Covid-19 vaccine in the United States.[194][195][196] |
| 2021 | March 11 | Clinical trial | Moderna starts clinical trial with the purpose to evaluate the safety and reactogenicity of 3 dose levels of mRNA-1283 and 1 dose level of mRNA-1273 vaccine given to healthy adults in 2 doses, 28 days apart, and 1 dose level of mRNA-1283 administered as a single dose to healthy adults.[197] | ||
| 2021 | March 29 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts clinical trial with the purpose to evaluate mRNA-1273.351 in naïve and previously vaccinated adults.[198] |
| 2021 | April 15 | Propionic acidemia | mRNA-3927 | Clinical trial | Moderna starts clinical trial with the purpose to evaluate investigational drug mRNA-3927 in participants with propionic acidemia.[199] |
| 2021 | April 16 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts a clinical trial to evaluate COVID-19 vaccine mRNA-1273 in adults with a kidney or liver solid organ transplant and in healthy adult participants. The primary goal of the study is to evaluate the safety of mRNA-1273 and the serum antibody (Ab) responses obtained 28 days after the last dose of mRNA-1273.[200] |
| 2021 | April 18 | COVID-19 | mRNA-1273 | Supply | Moderna partners with Asian pharmaceutical distributor Zuellig Pharma Corp. for mRNA-1273 vaccine distribution in the Philippines.[201][202] |
| 2021 | April 19 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts a clinical trial with the purpose to assess the comparative effectiveness and safety of approved SARS-CoV-2 vaccines in two cohorts of immunocompromised patients (HIV+ patients and transplant patients).[203] |
| 2021 | May 3 | COVID-19 | mRNA-1273 | Supply | Moderna signs with Gavi, the Vaccine Alliance an advance purchase agreement for 500 million doses of Covid-19 vaccine. The agreement is secured on behalf COVAX, a worldwide initiative aimed at equitable access to COVID-19 vaccines directed by the GAVI vaccine alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), alongside key delivery partner UNICEF.[204] |
| 2021 | May 28 | COVID-19 | mRNA-1273 | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate the immunogenicity and safety of mRNA-1273.211 vaccine for COVID-19 variants.[205] |
| 2021 | June 1 | COVID-19 | mRNA-1273 | Partnership | Moderna partners with Thermo Fisher Scientific to scale up COVID-19 vaccine production in the United States. Under the terms, Thermo Fisher agrees to provide with its commercial manufacturing site in Greenville, North Carolina to fill/finish manufacturing services and supply packaging for hundreds of millions of doses of the vaccine.[206][207] |
| 2021 | June 3 | Supply | Moderna signs pact with UNICEF for Covid-19 vaccine supply. UNICEF and its procurement partners, including the Pan American Health Organization, gains access to up to 34 million doses of Moderna’s COVID-19 vaccine, to be delivered in the fourth quarter 2021, with an option for up to 466 million doses in 2022.[208] | ||
| 2021 | June 8 | Zika virus | mRNA-1893 | Clinical trial | Moderna launches a clinical trial with the purpose to evaluate 2 dose levels of messenger RNA (mRNA)-1893 Zika vaccine in comparison to a placebo control in healthy participants who are flavivirus-seronegative and in participants who are flavivirus-seropositive.[209] |
| 2021 | July 6 | Team | Paul Burton becomes Moderna's chief marketing officer, joining the company after 16 years at Johnson & Johnson.[210] | ||
| 2021 | July 6 | Influenza | mRNA-1010 | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate mRNA-1010 seasonal influenza vaccine in healthy adults.[211] |
| 2021 | July 7 | COVID-19 | mRNA-1273 | Observational study | Moderna starts an observational study with the purpose to add to the ongoing active and passive safety signal detection through signal refinement and, if needed, evaluation of potential safety signals related to taking the SARS-CoV-2 mRNA-1273 vaccine.[212] |
| 2021 | July 12 | Team | Kate Cronin becomes Moderna's chief brand officer, joining the company from Ogilvy Health, part of WPP plc, where she served as Global CEO.[213][214][215] | ||
| 2021 | July 14 | Financial (valuation) | Moderna valuation Reaches US$100 billion.[216] | ||
| 2021 | June 18 | Cytomegalovirus | mRNA-1647 | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate the longer-term immune persistence of mRNA-1647 vaccine administered to CMV-seronegative and CMV-seropositive adults.[217] |
| 2021 | July 20 | COVID-19 | mRNA-1273 | Supply | Moderna partners with Takeda Pharmaceutical and the Ministry of Health, Labour and Welfare of Japan to supply additional 50 million doses of the Moderna COVID-19 vaccine and its updated variant booster vaccine candidate, if authorized, to begin delivery in 2022.[218] |
| 2021 | July 28 | Autoimmune diseases | mRNA‑6231 | Clinical trial | Moderna doses the first subject in Phase I clinical trial of its therapeutic candidate, mRNA-6231, for the treatment of patients with autoimmune diseases. The dose‑escalation study aims to analyze the safety, tolerability, pharmacokinetics and pharmacodynamics of the drug, which comprises a lipid nanoparticle (LNP)‑encapsulated messenger RNA encoding for mutein human interleukin-2 and adapted for improved regulatory T cell selectivity.[219][220][221] mRNA-6231 is Moderna's first subcutaneously administered therapeutic program.[222] |
| 2021 | August 6 | Clininal trial | Moderna starts clinical trial to evaluate mRNA-3705 in participants with isolated elevated methylmalonic acid (MMA) due to methylmalonyl-coenzyme A (CoA) mutase (MUT) deficiency.[223] mRNA-3705 is designed to instruct the body to restore the missing or dysfunctional proteins that cause MMA and consists of mRNA encoding human MUT enzyme encapsulated within a proprietary lipid nanoparticle (LNP).[224] | ||
| 2021 | August 10 | Infrastructure | Moderna announces a memorandum of understanding with the government of Canada to build an mRNA vaccine manufacturing plant at an unspecified location in the country. The company says the agreement would provide Canadians with access to domestically manufactured mRNA vaccines against respiratory viruses, including COVID-19, influenza, and respiratory syncytial virus, also laying the foundation for providing vaccines in a rapid pandemic response. Moderna also announces future similar partnerships with countries around the world.[225][226] | ||
| 2021 | August 23 | Methylmalonic acidemia | mRNA-3705 | Clinical trial | Moderna launches trial of mRNA vaccine for methylmalonic acidemia, announcing the first patient has been dosed in the Phase 1/2 study evaluating the safety and tolerability of mRNA-3705, its investigational mRNA therapeutic, administered via intravenous infusion in patients with isolated MMA due to MUT deficiency.[227] |
| 2021 | August 24 | COVID-19 | mRNA-1273 | Partnership | Moderna partners with National Football League and Major League Baseball teams to spread the message about its Covid-19 vaccine.[228][229] |
| 2021 | September 1 | COVID-19 | mRNA-1273 | Observational study | Moderna starts clinical trial to evaluate the outcomes of pregnancy in females exposed to the company's COVID-19 vaccine mRNA-1273 during pregnancy.[230] |
| 2021 | September 8 | Crigler-Najjar syndrome | mRNA-3351 | Partnership | Moderna partners with the Institute for Life Changing Medicines to develop a novel messenger RNA (mRNA) therapeutic (mRNA-3351) for Crigler-Najjar Syndrome Type 1 (CN-1). The purpose of the collaboration is to make an mRNA therapy for the treatment of CN-1 available at no cost to patients.[231][232] "Moderna Announces Advances Across Its Industry-Leading mRNA Pipeline and Provides Business Update"[233] |
| 2021 | September 9 | Glycogen storage disease | mRNA-3745 | Authorization | Moderna's drug candidate mRNA 3745 receives orphan drug status for glycogen storage disease type I in the United States.[234] An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. |
| 2021 | September 10 | COVID-19 | mRNA-1273 | Observational study | Moderna starts an observational study to evaluate the immunogenicity of a third dose of mRNA-1273 SARS-CoV-2 vaccine among cancer patients receiving the recommended third vaccine dose.[235] |
| 2021 | September 27 | Partnership | As part of a collaboration with Carnegie Mellon University, Moderna establishes its own Artificial Intelligence Academy. The initiative is expected to give the company's employees at all levels the education and empowerment necessary to identify and integrate machine learning and AI solutions into the company's systems and processes.[236] | ||
| 2021 | October 6 | Program launch | Moderna announces the launch of the Moderna Fellowship Program, an initiative aimed to "support the next generation of scientists and healthcare professionals as they innovate in the field of mRNA research towards improving patient care and population health".[237] | ||
| 2021 | October 10 | HIV | Partnership | Moderna partners with scientists at Scripps Research in La Jolla to use the company’s technology to make shots against HIV, after RNA vaccine technology proved to be safe and effective against the Covid-19.[238] | |
| 2021 | October 26 | Cytomegalovirus | mRNA-1647 | Clinical trial | Moderna initiates Phase III trial of its cytomegalovirus vaccine candidate mRNA-1647, which merges six messenger ribonucleic acids in a single vaccine that encode for two proteins found on the cytomegalovirus surface.[239][240] |
| 2021 | November 3 | Partnership | Moderna partners with gene-editing company Metagenomi in a research and development collaboration focused on advancing new gene editing systems for in vivo human therapeutic applications. Metagenomi provides its novel gene editing tools, aimed at leveraging Moderna’s mRNA platform, as well as lipid nanoparticle delivery technologies, with the goal of developing curative therapies for patients with serious genetic diseases.[241][242][243] | ||
| 2021 | November 5 | Chikungunya | mRNA-1944 | Clinical trial (shutdown) | Moderna announces interruption of its lead mRNA chikungunya candidate after phase 1, revealing it does not have plans to advance to a Phase 2 study.[244][245] |
| 2021 | November 8 | mRNA-1647 | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate the safety, reactogenicity, and immunogenicity of the mRNA-1647 vaccine administered according to a 3-study injection schedule in healthy cytomegalovirus (CMV)-seronegative and CMV-seropositive Japanese adults 18 to 40 years of age in the United States.[246] | |
| 2021 | November 9 | Propionic acidemia | mRNA-3927 | Clinical trial | Moderna initiates enrolment in a phase I/II Long-term extension trial of its candidate mRNA-3927 for propionic acidemia in infants, children, adolescents, adults, and the elderly) in the United States.[247] |
| 2021 | November 12 | mRNA-2752 | Clinical trial (result) | Moderna announces interim data from an ongoing Phase 1 clinical study of mRNA-2752 (Triplet) in patients with accessible solid tumors and lymphomas. The data shows that the company’s mRNA Ttriplet program given in combination with durvalumab is tolerated at all dose levels tested and elicited evidence of anti-tumor activity.[248] | |
| 2021 | November 12 | HIV | mRNA-1644 | Clinical trial | Moderna starts a Phase 1, randomized, first-in-human, open-label clinical trial with the purpose to evaluate eOD-GT8 60mer mRNA Vaccine (mRNA-1644) and Core-g28v2 60mer mRNA Vaccine (mRNA-1644v2-Core) in HIV-1 uninfected adults in good general health.[249] This is Moderna's clinical trial for HIV vaccine.[250][251][252][253] |
| 2021 | November 16 | Heart failure | AZD8601 | Clinical trial (result) | Moderna and AstraZeneca announce that the Phase 2a EPICCURE study of their jointly developed mRNA therapeutic AZD8601 has met the primary endpoint of safety and tolerability in patients with heart failure. AZD8601 encodes for vascular endothelial growth factor-A (VEGF-A).[254] |
| 2021 | November 17 | mRNA-1345 | Clinical trial | Moderna starts a clinical trial with the purpose to evaluate the mRNA-1345 vaccine and to demonstrate the efficacy of a single dose of mRNA-1345 vaccine in the prevention of a first episode of RSV-associated lower respiratory tract disease (RSV-LRTD) as compared with placebo from 14 days postinjection through 12 months. Over 33,000 participants would be enrolled.[255] | |
| 2021 | November 19 | Respiratory syncytial virus | mRNA-1345 | Clinical trial | Moderna doses the first subject in the Phase II/III trial of its respiratory syncytial virus (RSV) vaccine candidate, mRNA-1345, which encodes for a prefusion F glycoprotein, eliciting a superior neutralising antibody response. mRNA-1345 contains optimized protein and codon sequences, as well as uses lipid nanoparticle (LNP) similar to the Moderna's Covid-19 vaccine. The randomised, multicentre, placebo-controlled, observer-blind Phase II/III trial, named ConquerRSV, is planned to be conducted in many countries.[256] |
| 2021 | December 10 | COVID-19 | Supply | Moderna signs an agreement with Gavi, The Vaccine Alliance, for the supply of an additional 150 million COVID-19 vaccines, to be made available to COVAX at their lowest global tiered price. This amendment means a total of up to 650 million doses of the Moderna vaccine available to COVAX participants through 2021 and 2022.[257] | |
| 2021 | December 20 | COVID-19 | mRNA-1273 | Clinical trial (result) | Moderna releases data detailing the efficacy of its COVID-19 vaccine against the novel SARS-CoV-2 Omicron variant. Moderna's two-dose regimen is found to have relatively low levels of neutralizing antibodies effective against the variant. However, a 50 µg booster shot increases neutralizing antibody levels approximately 37-fold, while a 100 µg dose increases them approximately 83-fold.[258] |
| 2021 | December 28 | Epstein–Barr virus | mRNA-1189 | Clinical trial | Mopderna starts clinical trial with the purpose to evaluate the safety and reactogenicity of mRNA-1189 against Epstein–Barr virus infection in 18- to 30-year-old healthy adults.[259] |
| 2021 | Year round | COVID-19 | mRNA-1273 | Supply | Moderna ships approximately 800 million doses of its COVID-19 vaccine throughout the year.[260] |
| 2022 | COVID-19 | mRNA-1273 | Vaccine efficacy | Moderna CEO Stephane Bancel announces that the efficacy of Moderna COVID-19 vaccine boosters would likely decline over time, and some people may need a fourth shot to increase their protection.[261] | |
| 2022 | January 7 | Epstein-Barr Virus | mRNA-1189 | Research | Moderna announces the first participant has been dosed in the Phase 1 study of Epstein-Barr Virus (EBV) vaccine candidate mRNA-1189. EBV is a common viral infection with 83% of Americans seropositive by 19 years of age.[262][263] |
| 2022 | January 10 | Cancer | Partnership | Moderna partners with Philadelphia cell therapy developer Carisma Therapeutics to discover, develop and commercialize potential cancer treatments, using Carisma's in vivo engineered chimeric antigen receptor monocyte (CAR-M) technology. The collaboration includes Carisma receiving an upfront payment of US$45 million by Moderna, which would also invest US$35 million in Carisma in the form of a convertible note, and in return get options for up to 12 targets for potential cancer therapies. Carisma is a pioneer in cell therapies involving macrophages.[264][265][266] | |
| 2022 | January 10 | Drug pipeline | Moderna announces updates on its industry-leading mRNA pipeline, reporting 40 programs in development including 23 in ongoing clinical studies encompassing mRNA infectious disease vaccines and mRNA therapeutics spanning seven different modalities.[267] | ||
| 2022 | January 17 | COVID-19, influenza | Drug pipeline | Moderna announces development of a single shot that covers both flu and COVID-19, a vaccine that is not expected to be ready until the fall of 2023, and even then, it would only be available in some countries.[268] | |
| 2022 | January 27 | COVID-19 (Omicron variant | Clinical trial | Moderna announces trial launch aimed at studying the power of a redesigned booster shot for SARS-CoV-2 Omicron variant.[269] | |
| 2022 | January 28 | Glycogen storage disease | mRNA 3745 | Clinical trial | Moderna starts mRNA 3745 Phase-I clinical trials in Glycogen storage disease type I in the United States.[234][270] |
| 2022 | March 8 | Program launch | Moderna reveals its "global public health strategy", and announces it will provide access to the technology to produce its mRNA COVID-19 vaccine to countries that are most in need. The company says that it would not use its patents to block manufacturing of mRNA vaccines as long as the shots are used strictly within the countries.[271] | ||
| 2022 | March 10 | Partnership | Researchers at the University of Queensland partner with Moderna to develop vaccines against diseases such as tuberculosis, dengue, malaria, Zika and HIV.[272] | ||
| 2022 | March 24 | mRNA-1230, mRNA-1287 | Drug pipeline | During its Vaccines Day, Moderna announces new vaccination programs, including the development of mRNA-1230 vaccine to cover SARS-CoV-2, influenza, and respiratory syncytial virus (RSV) as well as another candidate, mRNA-1287, for endemic human coronaviruses: HCoV-229E, -NL63, -OC43 and -HKU1.[273] | |
| 2022 | March 26 | Partnership | McGill University joins Moderna's mRNA Access program, which focuses on facilitating collaborative research and preclinical development to accelerate innovation on new vaccines and medicines that address existing and emerging diseases.[274][275] |
Numerical and visual data
Google Trends
The chart below shows Google Trends data for Moderna (Biotechnology Company), from January 2010 to January 2022, when the screenshot was taken. Interest is also ranked by country and displayed on world map. [276] The first increase in early 2020 happens as COVID-19 starts spreading and Moderna announces its COVID-19 vaccine efforts. Further increases near the end of 2020 occur once Moderna succeeds in its human clinical trials of the COVID-19 vaccine. After that, interest in Moderna largely tracks the evolution of the COVID-19 pandemic.
Google Finance
The image below shows Moderna stock valuation in USD from IPO to January 14, 2022, when the screenshot was taken.[277]
Wikipedia Views
The chart below shows pageviews of the English Wikipedia article Moderna, from July 2015 to December 2021.[278]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by User:Sebastian.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
Timeline update strategy
See also
External links
References
- ↑ 1.0 1.1 "Moderna is developing three new mRNA-based vaccines for seasonal flu, HIV and Nipah virus". TechCrunch. Retrieved 19 January 2022.
- ↑ 2.0 2.1 "Moderna's top scientist steps down amid a billion-dollar R&D push". FierceBiotech. Retrieved 1 February 2022.
- ↑ "Moderna funding rounds, valuation and investors". craft. Retrieved 11 April 2022.
- ↑ "Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine". STAT. 10 January 2017. Retrieved 3 February 2022.
- ↑ "Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine". STAT. 10 January 2017. Retrieved 3 February 2022.
- ↑ October 29th, reas Ebertz 15 April 2021 (15 April 2021). "The history of mRNA applications". The DNA Universe BLOG. Retrieved 12 March 2022.
- ↑ "Fact check: Dr. Fauci was not the first CEO and other false claims about biotech company Moderna". Reuters. 1 September 2020. Retrieved 30 March 2022.
- ↑ Rother, K.; Potrzebowski, W.; Puton, T.; Rother, M.; Wywial, E.; Bujnicki, J. M. (1 March 2012). "A toolbox for developing bioinformatics software". Briefings in Bioinformatics. 13 (2): 244–257. doi:10.1093/bib/bbr035.
- ↑ 9.0 9.1 "Ego, ambition, and turmoil: Inside one of biotech's most secretive startups". STAT. 13 September 2016. Retrieved 19 January 2022.
- ↑ Pollack, Andrew (21 March 2013). "AstraZeneca Makes a Bet on an Untested Technique". The New York Times. Retrieved 19 January 2022.
- ↑ Staff, Robert Weisman Globe. "Moderna in line for $240m licensing deal - The Boston Globe". BostonGlobe.com. Retrieved 19 January 2022.
- ↑ says, IP Guy (21 March 2013). "Xconomy: AstraZeneca Shells Out $240M Upfront For Moderna mRNA Drugs". Xconomy. Retrieved 19 January 2022.
- ↑ Therapeutics, Moderna. "Industry Leader John Reynders Joins Moderna Therapeutics as Chief Information Officer". www.prnewswire.com. Retrieved 31 March 2022.
- ↑ "Moderna lands $25M grant to develop its RNA platform against infectious diseases, bioterror". FierceBiotech. Retrieved 19 January 2022.
- ↑ "Xconomy: Moderna Snags $25M DARPA Grant to Fight Pandemics With mRNA Drugs". Xconomy. 2 October 2013. Retrieved 19 January 2022.
- ↑ Therapeutics, Moderna. "DARPA Awards Moderna Therapeutics A Grant For Up To $25 Million To Develop Messenger RNA Therapeutics™". www.prnewswire.com. Retrieved 8 February 2022.
- ↑ Therapeutics, Moderna. "Moderna Therapeutics Announces Expansion to New Headquarters and Laboratories at 200 Tech Square". www.prnewswire.com. Retrieved 19 January 2022.
- ↑ "MLSC tax incentive company Moderna Therapeutics announces expansion to new headquarters and laboratories at 200 Tech Square". MLSC. 17 October 2013. Retrieved 8 February 2022.
- ↑ Schwartz, Heidi (24 October 2013). "Moderna Therapeutics Expands In New HQ And Lab In Cambridge, MA". Business Facilities Magazine. Retrieved 8 February 2022.
- ↑ "Xconomy: Moderna Vacuums Up Another $110M to Make Messenger RNA Drugs". Xconomy. 20 November 2013. Retrieved 18 January 2022.
- ↑ "Alexion Pharmaceuticals and Moderna Therapeutics Announce Exclusive Strategic Agreement to Develop Messenger RNA Therapeutics™ for Rare Diseases | Alexion Pharmaceuticals, Inc.". ir.alexion.com. Retrieved 19 January 2022.
- ↑ "Xconomy: Moderna, Living a Charmed Life, Pulls in Another $125M From Alexion". Xconomy. 13 January 2014. Retrieved 19 January 2022.
- ↑ "Alexion, Moderna announce agreement to develop messenger RNA therapeutics". www.boston.com. Retrieved 18 January 2022.
- ↑ "Moderna appoints biotechnology investment banking veteran Lorence Kim as chief financial officer". www.thepharmaletter.com. Retrieved 19 January 2022.
- ↑ Therapeutics, Moderna. "Moderna Appoints Biotechnology Investment Banking Veteran Lorence Kim as Chief Financial Officer". www.prnewswire.com. Retrieved 8 February 2022.
- ↑ "Moderna Appoints Biotech Investment Banking Veteran Lorence Kim as CFO". Wall Street Journal. 6 March 2014. Retrieved 8 February 2022.
- ↑ Herper, Matthew. "Moderna Therapeutics Raises $450 Million In Biggest Biotech Private Round Ever". Forbes. Retrieved 1 February 2022.
- ↑ Gormley, Brian (5 January 2015). "Moderna Therapeutics Raises Record $450M for Messenger RNA Drugs". Wall Street Journal. Retrieved 1 February 2022.
- ↑ Timmerman, Luke. "Merck Joins Messenger RNA Frenzy, Betting $100M On Moderna Therapeutics". Forbes. Retrieved 1 February 2022.
- ↑ "Xconomy: More Green For Moderna as Merck Makes $50M Equity Grab, mRNA Deal". Xconomy. 13 January 2015. Retrieved 1 February 2022.
- ↑ "Bay State is Home to Four Biotech Unicorns". BioSpace. Retrieved 1 April 2022.
- ↑ "Moderna and Institut Pasteur Sign Strategic Research Collaboration". Institut Pasteur. 6 February 2015. Retrieved 9 February 2022.
- ↑ Tirrell, Meg (12 May 2015). "The biotech targeting personalized medicine". CNBC. Retrieved 1 February 2022.
- ↑ "Moderna Therapeutics, Inc. Appoints Tal Zaks, Former Head Of Sanofi Global Oncology, As Chief Medical Officer". BioSpace. Retrieved 8 February 2022.
- ↑ Therapeutics, Moderna. "Moderna Appoints Tal Zaks, Former Head of Sanofi Global Oncology, as Chief Medical Officer". www.prnewswire.com. Retrieved 8 February 2022.
- ↑ "Moderna joins the personalized cancer vaccine rush with third new venture". FierceBiotech. Retrieved 1 February 2022.
- ↑ "Trial Details". trials.modernatx.com. Retrieved 10 February 2022.
- ↑ "A Phase 1, First-in-Human, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety and Immunogenicity of H10N8 Antigen mRNA in Healthy Adult Subjects". clinicaltrials.gov. clinicaltrials.gov. 28 December 2018. Retrieved 19 February 2022.
- ↑ "Moderna Therapeutics Announces Transition to a Clinical Stage Company, Provides Business Update and Outlines 2016 Strategic Priorities". www.businesswire.com. 11 January 2016. Retrieved 2 February 2022.
- ↑ "Moderna Announces Transition to a Clinical Stage Company, Provides…". Flagship Pioneering. Retrieved 9 February 2022.
- ↑ "AstraZeneca and Moderna Therapeutics announce new collaboration to co-develop and co-commercialise immuno-oncology mRNA therapeutics™". www.astrazeneca.com. Retrieved 10 February 2022.
- ↑ 42.0 42.1 42.2 "Key Milestones and Advancements in mRNA Technology - Moderna". www.modernatx.com. Retrieved 10 February 2022.
- ↑ Writer, GEN Staff (12 January 2016). "Moderna Wins Initial $20M Grant from Gates Foundation". GEN - Genetic Engineering and Biotechnology News. Retrieved 1 February 2022.
- ↑ "Gates Foundation Invests $20 Million to Advance HIV Care". philanthropynewsdigest.org. Retrieved 11 February 2022.
- ↑ "Research not fit to print". Nature Biotechnology. February 2016. pp. 115–115. doi:10.1038/nbt.3488. Retrieved 19 January 2022.
- ↑ "Moderna Therapeutics appoints chief business officer". PMLive. 12 April 2016. Retrieved 2 February 2022.
- ↑ "Moderna Therapeutics Names CBO". pink.pharmaintelligence.informa.com. Retrieved 9 February 2022.
- ↑ "Saqib Islam to become CBO at Moderna Therapeutics | MedNous". www.mednous.com. Retrieved 9 February 2022.
- ↑ "A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study to Evaluate the Safety and Immunogenicity of VAL-339851 in Healthy Subjects". clinicaltrials.gov. clinicaltrials.gov. 7 January 2021. Retrieved 19 February 2022.
- ↑ "Moderna Therapeutics and Charles River Laboratories Announce Strategic Collaboration to Scale Moderna's Nonclinical Development Efforts for Novel mRNA Therapeutics | Charles River Laboratories International, Inc.". ir.criver.com. Retrieved 10 February 2022.
- ↑ "Charles River signs up to help Moderna with mRNA pipeline". FierceBiotech. Retrieved 13 February 2022.
- ↑ Writer, GEN Staff (29 June 2016). "Merck, Moderna to Develop mRNA-Based Personalized Cancer Vaccines". GEN - Genetic Engineering and Biotechnology News. Retrieved 1 February 2022.
- ↑ "Merck and Moderna Announce Strategic Collaboration to Advance Novel mRNA-Based Personalized Cancer Vaccines with KEYTRUDA® (pembrolizumab) for the Treatment of Multiple Types of Cancer". Merck.com. Retrieved 9 February 2022.
- ↑ "Moderna Enters $200M+ Strategic Collaboration and License Agreement with Merck". www.goodwinlaw.com. Retrieved 9 February 2022.
- ↑ "UPDATED: Vertex, Moderna pair to create mRNA cystic fibrosis treatment". FierceBiotech. Retrieved 1 February 2022.
- ↑ "Cash-rich Moderna to begin first AstraZeneca trial under collab deal". FierceBiotech. Retrieved 1 February 2022.
- ↑ "Moderna Therapeutics Provides Mid-Year Corporate Update, Announces Continued Clinical Development Progress and Pipeline Acceleration". www.businesswire.com. 26 July 2016. Retrieved 2 February 2022.
- ↑ "Vascular Endothelial Growth Factor A". sciencedirect.com. Retrieved 14 February 2022.
- ↑ Esserman, Laura (15 January 2022). "Testing the Ability of Pembrolizumab and mRNA 2752 to Alter the Tumor Immune MicroEnvionment (TIME) of High Risk DCIS". clinicaltrials.gov. clinicaltrials.gov. Retrieved 19 February 2022.
- ↑ "Moderna Announces Funding Award from BARDA for $8 Million with Potential of up to $125 Million to Accelerate Development of Zika Messenger RNA (mRNA) Vaccine". www.businesswire.com. 7 September 2016. Retrieved 1 February 2022.
- ↑ Writer, GEN Staff (7 September 2016). "Moderna Wins Up to $125M from BARDA Toward Zika mRNA Vaccine". GEN - Genetic Engineering and Biotechnology News. Retrieved 2 February 2022.
- ↑ "Moderna to plow $110M into manufacturing build-out". FierceBiotech. Retrieved 1 February 2022.
- ↑ September 30. "Moderna Therapeutics to Build $110M Plant in Norwood". GlobeSt. Retrieved 2 February 2022.
- ↑ Times, Boston Real Estate (6 October 2016). "DPS and TRIA Selected to Design 200,000 SF GMP mRNA Clinical Manufacturing Facility for Moderna Therapeutics". Boston Real Estate Times. Retrieved 2 February 2022.
- ↑ "Moderna taps Catalent for clinical manufacturing". BioPharma Dive. Retrieved 2 February 2022.
- ↑ "A Phase 1, Randomized, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety and Immunogenicity of mRNA 1325 Zika Vaccine in Healthy Adults in a Non-endemic Zika Region". clinicaltrials.gov. clinicaltrials.gov. 6 December 2019. Retrieved 19 February 2022.
- ↑ "Moderna Joins the Human Vaccines Project to Help Advance Fundamental Understanding of the Immune System" (PDF). modernatx.com. Retrieved 16 February 2022.
- ↑ Herper, Matthew. "Mysterious $5 Billion Biotech Firm Moderna Lays Out Drug Pipeline". Forbes. Retrieved 2 February 2022.
- ↑ "Moderna Provides Pipeline and Full-Year Corporate Update". www.businesswire.com. 9 January 2017. Retrieved 9 February 2022.
- ↑ "Moderna Provides Pipeline and Full-Year Corporate Update". Biotech Finances (in français). 10 January 2017. Retrieved 10 February 2022.
- ↑ Rockoff, Jonathan D. (27 April 2017). "Startup Moderna Shows Promise in Vaccine Trial". Wall Street Journal. Retrieved 3 February 2022.
- ↑ "Moderna Announces Positive Interim Phase 1 Clinical Data Demonstrating First mRNA Vaccine Candidate, mRNA-1440, Induces High Levels of Immunogenicity" (PDF). modernatx.com. Retrieved 9 February 2022.
- ↑ "Secretive Moderna reveals first-in-human data from mRNA vaccine tech". FiercePharma. Retrieved 2 February 2022.
- ↑ Vardi, Nathan. "Moderna Can't Escape My Intellectual Property, Says Arbutus CEO". Forbes. Retrieved 3 February 2022.
- ↑ "Publication in Cell Demonstrates Moderna's Zika mRNA Vaccine Prevents In Utero Transmission of Zika Virus in Mice, Protects Against Zika-Related Congenital Damage" (PDF). modernatx.com. Retrieved 17 February 2022.
- ↑ Richner, Justin M.; Jagger, Brett W.; Shan, Chao; Fontes, Camila R.; Dowd, Kimberly A.; Cao, Bin; Himansu, Sunny; Caine, Elizabeth A.; Nunes, Bruno T.D.; Medeiros, Daniele B.A.; Muruato, Antonio E.; Foreman, Bryant M.; Luo, Huanle; Wang, Tian; Barrett, Alan D.; Weaver, Scott C.; Vasconcelos, Pedro F.C.; Rossi, Shannan L.; Ciaramella, Giuseppe; Mysorekar, Indira U.; Pierson, Theodore C.; Shi, Pei-Yong; Diamond, Michael S. (July 2017). "Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease". Cell. 170 (2): 273–283.e12. doi:10.1016/j.cell.2017.06.040.
- ↑ "Key partner cuts ties with brash biotech startup Moderna, raising big questions about its pipeline". STAT. 27 July 2017. Retrieved 19 January 2022.
- ↑ "Alexion leaves Moderna, others behind in R&D shake-up". BioPharma Dive. Retrieved 2 February 2022.
- ↑ "Moderna Hires Senior VP of Late Stage Technical Development, Manufacturing". www.pharmoutsourcing.com. Retrieved 9 February 2022.
- ↑ "Moderna brings on former Novartis executive to oversee manufacturing of mRNA therapies". FiercePharma. Retrieved 2 February 2022.
- ↑ "Moderna taps Novartis exec to lead manufacturing blitz". www.bizjournals.com. Retrieved 3 February 2022.
- ↑ "A Phase 1/2, Open-Label, Multicenter, Dose Escalation and Efficacy Study of mRNA-2416, a Lipid Nanoparticle Encapsulated mRNA Encoding Human OX40L, for Intratumoral Injection Alone or in Combination With Durvalumab for Patients With Advanced Malignancies". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 19 February 2022.
- ↑ "A Phase 1, Open-Label, Multicenter Study to Assess the Safety, Tolerability, and Immunogenicity of mRNA-4157 Alone in Subjects With Resected Solid Tumors and in Combination With Pembrolizumab in Subjects With Unresectable Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 12 February 2022.
- ↑ "Moderna initiates Chikungunya vaccine human test, replaces backup Zika candidate". FiercePharma. Retrieved 3 February 2022.
- ↑ "Trial Details". trials.modernatx.com. Retrieved 10 February 2022.
- ↑ "A Phase 1, Randomized, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety and Immunogenicity of VAL-181388 in Healthy Adults in a Non Endemic Chikungunya Region". clinicaltrials.gov. clinicaltrials.gov. 16 January 2020. Retrieved 19 February 2022.
- ↑ "Moderna Ends "Venture" Experiment, Dissolves Four Startups". xconomy.com. Retrieved 2 February 2022.
- ↑ "Moderna changes up R&D model, posts cancer, CV trial updates". FierceBiotech. Retrieved 2 February 2022.
- ↑ "A Phase 1/2, Open-Label, Multicenter, Dose Escalation and Efficacy Study of mRNA-2416, a Lipid Nanoparticle Encapsulated mRNA Encoding Human OX40L, for Intratumoral Injection Alone or in Combination With Durvalumab for Patients With Advanced Malignancies". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 10 February 2022.
- ↑ "AstraZeneca, Moderna pen deal for mRNA heart failure drug". FierceBiotech. Retrieved 2 February 2022.
- ↑ Fernández, Clara Rodríguez (2 November 2017). "AstraZeneca and Moderna want to beat Novartis at Treating Heart Failure". Labiotech.eu. Retrieved 3 February 2022.
- ↑ Sedic, Maja; Senn, Joseph J.; Lynn, Andy; Laska, Michael; Smith, Mike; Platz, Stefan J.; Bolen, Joseph; Hoge, Stephen; Bulychev, Alex; Jacquinet, Eric; Bartlett, Victoria; Smith, Peter F. (March 2018). "Safety Evaluation of Lipid Nanoparticle–Formulated Modified mRNA in the Sprague-Dawley Rat and Cynomolgus Monkey". Veterinary Pathology. 55 (2): 341–354. doi:10.1177/0300985817738095.
- ↑ "Herpes Vaccine: Despite Setbacks, There is Still Hope". BioSpace. Retrieved 4 February 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of Cytomegalovirus Vaccines mRNA-1647 and mRNA-1443 When Administered to Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 13 January 2021. Retrieved 19 February 2022.
- ↑ 95.0 95.1 "Moderna again uses J.P. Morgan as stage for pipeline update, including for vaccines". FiercePharma. Retrieved 3 February 2022.
- ↑ "Trial Details". trials.modernatx.com. Retrieved 10 February 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1653, a Combined Human Metapneumovirus and Human Parainfluenza Virus Type 3 Vaccine, When Administered to Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 5 February 2020. Retrieved 19 February 2022.
- ↑ "Moderna Announces an Array of Clinical Advances and Outlines 2018 Priorities; 19 Development Candidates, including 10 Clinical". www.bloomberg.com. Retrieved 10 February 2022.
- ↑ "Moderna raises $500M to move mRNA drugs deeper into human tests". FierceBiotech. Retrieved 3 February 2022.
- ↑ Herper, Matthew. "Despite Doubters, Moderna Raises $500 Million, Is Now Worth $7 Billion". Forbes. Retrieved 3 February 2022.
- ↑ "Moderna, Still Private, Scoops Up Yet Another $500M". xconomy.com. Retrieved 3 February 2022.
- ↑ "Moderna's vaccines head steps down as multiple programs undergo phase 1: report". FiercePharma. Retrieved 3 February 2022.
- ↑ "Moderna's head of vaccines quits after a pivot to vaccines". STAT. 6 March 2018. Retrieved 11 February 2022.
- ↑ "Trial Details". trials.modernatx.com. Retrieved 10 February 2022.
- ↑ "A Longitudinal, Exploratory, Natural History Study to Further Characterize and Describe the Signs and Symptoms of Patients With Organic Acidemias". clinicaltrials.gov. clinicaltrials.gov. 29 July 2021. Retrieved 19 February 2022.
- ↑ "Merck to invest $125m in Moderna Therapeutics - Pharmaceutical Business review". www.pharmaceutical-business-review.com. Retrieved 11 February 2022.
- ↑ Writer, GEN Staff (4 May 2018). "Merck Invests $125M in Moderna in Expanded mRNA Cancer Vaccine Partnership". GEN - Genetic Engineering and Biotechnology News. Retrieved 3 February 2022.
- ↑ "Merck partners with Moderna to test KRas cancer vaccines in humans". cen.acs.org. Retrieved 3 February 2022.
- ↑ Herper, Matthew. "Merck Teams With Moderna To Take On One Of The Toughest Targets In Cancer". Forbes. Retrieved 3 February 2022.
- ↑ "Moderna's $110M Norwood site built with expansion hopes". www.bizjournals.com. Retrieved 18 January 2022.
- ↑ Molteni, Megan. "Making Personalized Cancer Vaccines Takes an Army—of Robots". Wired. Retrieved 18 January 2022.
- ↑ "Moderna Opens New Manufacturing Site in Norwood, MA". www.businesswire.com. 17 July 2018. Retrieved 3 February 2022.
- ↑ "Moderna opens $110M manufacturing site for its mRNA program". FiercePharma. Retrieved 3 February 2022.
- ↑ "Moderna Named a Top Employer by Science for the Fourth Consecutive Year". AP NEWS. 17 December 2021. Retrieved 3 February 2022.
- ↑ "A Phase 1, Open-Label, Multicenter, Dose Escalation Study of mRNA-2752, a Lipid Nanoparticle Encapsulating mRNAs Encoding Human OX40L, IL-23, and IL-36γ, for Intratumoral Injection Alone and in Combination With Immune Checkpoint Blockade". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna Had the Biggest Biotech IPO Ever. Here's What That Says About the Industry's Future". Fortune. Retrieved 18 January 2022.
- ↑ Pflanzer, Lydia Ramsey. "Moderna just priced the biggest IPO in biotech history, valuing the startup at $7.5 billion". Business Insider. Retrieved 18 January 2022.
- ↑ "Moderna Announces Pricing of Initial Public Offering". www.businesswire.com. 7 December 2018. Retrieved 18 January 2022.
- ↑ "Moderna Therapeutics Sets Record for Biggest Biotech IPO". BioSpace. Retrieved 3 February 2022.
- ↑ Toy, Sarah. "Moderna: 5 things to know about the largest biotech to IPO". MarketWatch. Retrieved 3 February 2022.
- ↑ "Moderna Announces Recent Progress in Its Immuno-Oncology and Rare Disease Programs and Highlights Corporate Objectives". www.businesswire.com. 8 January 2019. Retrieved 3 February 2022.
- ↑ "Moderna CEO makes case for firm's lofty valuation". PMLive. 9 January 2019. Retrieved 3 February 2022.
- ↑ "A Phase 1, Randomized, Placebo-Controlled, Dose Ranging Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of mRNA-1944, Encoding for an Anti-Chikungunya Virus Monoclonal Antibody, in Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 5 July 2021. Retrieved 19 February 2022.
- ↑ "Moderna Announces Dosing of the First Monoclonal Antibody Encoded by mRNA in a Clinical Trial". www.businesswire.com. 5 February 2019. Retrieved 9 February 2022.
- ↑ "Bloomberg - Are you a robot?". www.bloomberg.com. Retrieved 9 February 2022.
- ↑ "Moderna Announces Positive Interim Phase 1 Data for First Combination Vaccine Against the Respiratory Viruses hMPV and PIV3". BioSpace. Retrieved 12 February 2022.
- ↑ "Phase 1 Data Published in Nature Communications Show Potential of mRNA Encoding VEGF-A as a Regenerative Therapeutic". www.businesswire.com. 20 February 2019. Retrieved 4 February 2022.
- ↑ Times, Boston Real Estate (3 April 2019). "Moderna's Clinical Manufacturing Facility Wins 2019 Facility of the Future Award". Boston Real Estate Times. Retrieved 4 February 2022.
- ↑ "Moderna Publishes Influenza Vaccine Clinical Trial Data". BioSpace. Retrieved 3 February 2022.
- ↑ "Chikungunya Vaccine Candidate Launches Phase 1 Study". www.precisionvaccinations.com. Retrieved 9 February 2022.
- ↑ "Trial Details". trials.modernatx.com. Retrieved 10 February 2022.
- ↑ "A Global, Phase 1/2, Open Label, Dose Escalation Study to Evaluate the Safety, Pharmacodynamics, and Pharmacokinetics of mRNA-3704 in Patients With Isolated Methylmalonic Acidemia Due to Methylmalonyl-CoA Mutase Deficiency". clinicaltrials.gov. clinicaltrials.gov. 10 November 2020. Retrieved 19 February 2022.
- ↑ "A Phase 1, Open-Label, Multicenter Study to Assess the Safety and Tolerability of mRNA-5671/V941 as a Monotherapy and in Combination With Pembrolizumab in Participants With KRAS Mutant Advanced or Metastatic Non-Small Cell Lung Cancer, Colorectal Cancer or Pancreatic Adenocarcinoma". clinicaltrials.gov. clinicaltrials.gov. 9 February 2022. Retrieved 18 February 2022.
- ↑ "A Phase 2 Randomized Study of Adjuvant Immunotherapy With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab Versus Pembrolizumab Alone After Complete Resection of High-Risk Melanoma (KEYNOTE- 942)". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety, Tolerability, and Immunogenicity of Zika Vaccine mRNA-1893 in Healthy Flavivirus Seropositive and Seronegative Adults". clinicaltrials. clinicaltrials.gov. 20 April 2021. Retrieved 31 March 2022.
- ↑ "Moderna Receives FDA Fast Track Designation for Zika Vaccine mRNA-1893". BioSpace. Retrieved 3 February 2022.
- ↑ "Moderna Appoints Tracey Franklin as Chief Human Resources Officer". www.businesswire.com. 4 September 2019. Retrieved 3 February 2022.
- ↑ "Cytomegalovirus Vaccine Candidate Heads to Phase 2 Study". www.precisionvaccinations.com. Retrieved 4 February 2022.
- ↑ "Moderna Announces Positive Phase 1 Results for the First Systemic Messenger RNA Therapeutic Encoding a Secreted Protein (mRNA-1944)". www.businesswire.com. 12 September 2019. Retrieved 9 February 2022.
- ↑ "Moderna to expand mRNA plant - Bioprocess Insider". BioProcess International. 2 October 2019. Retrieved 3 February 2022.
- ↑ "Moderna plans to build mRNA vaccine manufacturing plant in Africa". devex.com. Retrieved 11 February 2022.
- ↑ "Moderna Receives FDA Fast Track Designation for Propionic Acidemia Program (mRNA-3927)". www.businesswire.com. 22 October 2019. Retrieved 3 February 2022.
- ↑ Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review. [1]
- ↑ "Moderna Named Top Employer by Science for Fifth Consecutive Year". www.businesswire.com. 24 October 2019. Retrieved 3 February 2022.
- ↑ "A Phase 1b, Randomized, Observer-Blind, Placebo-Controlled, Dose Ranging Trial to Evaluate the Safety and Immunogenicity of mRNA-1653, a Combined Human Metapneumovirus (hMPV) and Parainfluenza Virus Type 3 (PIV3) Vaccine When Administered to Adults, and to Children 12 to 59 Months of Age With Serologic Evidence of Prior Exposure". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 19 February 2022.
- ↑ "A Phase 2, Randomized, Observer-Blind, Placebo-Controlled, Dose-Finding Trial to Evaluate the Safety and Immunogenicity of Cytomegalovirus Vaccine mRNA-1647 in Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna Announces Funding Award from CEPI to Accelerate Development of Messenger RNA (mRNA) Vaccine Against Novel Coronavirus". www.businesswire.com. 23 January 2020. Retrieved 4 February 2022.
- ↑ "CEPI to fund three programmes to develop vaccines against the novel coronavirus, nCoV-2019". CEPI. Retrieved 13 February 2022.
- ↑ "Moderna Announces First Patient Enrolled in Phase 1/2 Study of mRNA-3704 for Methylmalonic Acidemia". www.bloomberg.com. Retrieved 12 February 2022.
- ↑ "Moderna enrols first patient in Phase I/II trial of mRNA-3704". Clinical Trials Arena. 11 February 2020. Retrieved 10 February 2022.
- ↑ "Phase I, Open-Label, Dose-Ranging Study of the Safety and Immunogenicity of 2019-nCoV Vaccine (mRNA-1273) in Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 10 February 2022. Retrieved 19 February 2022.
- ↑ Correspondent, Jeremy C. Fox Globe; May 1, Updated. "Moderna partners with Swiss firm Lonza to produce potential coronavirus vaccine - The Boston Globe". BostonGlobe.com. Retrieved 4 February 2022.
- ↑ "Moderna Doses First Patients in Phase 2 Study of COVID-19 Vaccine". PharmaNewsIntelligence. 4 June 2020. Retrieved 13 February 2022.
- ↑ "Moderna Announces First Participants in Each Age Cohort Dosed in Phase 2 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus". investors.modernatx.com. Retrieved 13 February 2022.
- ↑ "A Phase 2a, Randomized, Observer-Blind, Placebo Controlled, Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 SARS-COV-2 Vaccine in Adults Aged 18 Years and Older". clinicaltrials.gov. clinicaltrials.gov. 27 November 2021. Retrieved 19 February 2022.
- ↑ Minaya, Ezequiel. "Moderna Hires New CFO Amid Race To Develop Coronavirus Treatment". Forbes. Retrieved 9 February 2022.
- ↑ "Moderna Partners with Catalent to Deepen COVID-19". cen.acs.org. Retrieved 4 February 2022.
- ↑ "Moderna and Catalent Announce Collaboration for Fill-Finish Manufacturing of Moderna's COVID-19 Vaccine Candidate". Catalent. 25 June 2020. Retrieved 17 February 2022.
- ↑ Jackson, Lisa A.; Anderson, Evan J.; Rouphael, Nadine G.; Roberts, Paul C.; Makhene, Mamodikoe; Coler, Rhea N.; McCullough, Michele P.; Chappell, James D.; Denison, Mark R.; Stevens, Laura J.; Pruijssers, Andrea J. (July 14, 2020). "An mRNA Vaccine against SARS-CoV-2—Preliminary Report". New England Journal of Medicine. 383 (20): 1920–1931. ISSN 0028-4793. doi:10.1056/NEJMoa2022483.
At the 100-microgram dose, the one Moderna is advancing into larger trials, all fifteen patients experienced side effects, including fatigue, chills, headache, muscle pain, and pain at the site of injection. All side effects were considered mild or moderate. A higher, 250-microgram dose led to more serious reactions and has been set aside.
- ↑ "Moderna gets further $472 million U.S. award for coronavirus vaccine development". Reuters. 26 July 2020. Retrieved 9 February 2022.
- ↑ "A Phase 3, Randomized, Stratified, Observer-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety, and Immunogenicity of mRNA-1273 SARS-CoV-2 Vaccine in Adults Aged 18 Years and Older". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "HHS, DoD to Manufacture 100M Doses of Moderna's COVID-19 Vaccine". PharmaNewsIntelligence. 13 August 2020. Retrieved 12 February 2022.
- ↑ "Trump Administration collaborates with Moderna to produce 100 million doses of COVID-19 investigational vaccine". public3.pagefreezer.com. Retrieved 12 February 2022.
- ↑ "Moderna and Vertex Establish New Collaboration to Treat Cystic Fibrosis Using Gene Editing". www.businesswire.com. 16 September 2020. Retrieved 4 February 2022.
- ↑ "Moderna and Vertex Establish New Collaboration to Treat Cystic Fibrosis Using Gene Editing". www.bloomberg.com. Retrieved 13 February 2022.
- ↑ "Moderna and Vertex partner on gene-editing therapies for cystic fibrosis". Pharmaceutical Technology. 17 September 2020. Retrieved 13 February 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Placebo-Controlled, Dose Escalation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1345, an mRNA Vaccine Targeting Respiratory Syncytial Virus (RSV), in Healthy Younger Adults Aged 18 to 49 Years, Women of Child-Bearing Potential Aged 18 to 40 Years, Healthy Older Adults Aged 65 to 79 Years, Japanese Older Adults Aged ≥ 60 Years, and RSV-Seropositive Children Aged 12 to 59 Months". clinicaltrials.gov. clinicaltrials.gov. 27 January 2022. Retrieved 19 February 2022.
- ↑ "Takeda Expands COVID-19 Vaccine Supply in Japan Through Partnership with Moderna and Government of Japan". www.takeda.com. Retrieved 12 February 2022.
- ↑ "Moderna partners with Chiesi on PAH research". www.thepharmaletter.com. Retrieved 4 February 2022.
- ↑ "Second Analysis Finds Moderna's COVID-19 Vaccine 94.1% Effective". PharmaNewsIntelligence. 1 December 2020. Retrieved 12 February 2022.
- ↑ "Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ "Moderna and UK sign deal to supply two million more Covid-19 vaccine doses". Pharmaceutical Technology. 30 November 2020. Retrieved 4 February 2022.
- ↑ "A Phase 2/3, Randomized, Observer-Blind, Placebo-Controlled Study to Evaluate the Safety, Reactogenicity, and Effectiveness of mRNA-1273 SARS-CoV-2 Vaccine in Healthy Adolescents 12 to". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna Launches Study of COVID-19 Vaccine in Adolescents". PharmaNewsIntelligence. 15 December 2020. Retrieved 12 February 2022.
- ↑ "Moderna Announces First Participants Dosed in Phase 2/3 Study of COVID-19 Vaccine Candidate in Adolescents". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ Dwyer, Colin (18 December 2020). "Moderna's COVID-19 Vaccine Becomes 2nd To Earn FDA Authorization". NPR. Retrieved 4 February 2022.
- ↑ "FDA grants emergency authorization to Moderna's Covid-19 vaccine". NBC News. Retrieved 4 February 2022.
- ↑ "Moderna becomes second COVID-19 vaccine to receive US approval". euronews. 19 December 2020. Retrieved 9 February 2022.
- ↑ "Moderna's Covid-19 vaccine obtains Health Canada authorisation". Pharmaceutical Technology. 24 December 2020. Retrieved 4 February 2022.
- ↑ "Israeli Ministry of Health Authorizes COVID-19 Vaccine Moderna for Use in Israel". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ "Moderna Provides COVID-19 Vaccine Supply Update". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ "Moderna Ups COVID-19 Vaccine Manufacturing Estimate to 600M in 2021". PharmaNewsIntelligence. 5 January 2021. Retrieved 12 February 2022.
- ↑ "EU Grants Emergency Use for Moderna's mRNA COVID-19 Vaccine". PharmaNewsIntelligence. 11 January 2021. Retrieved 12 February 2022.
- ↑ "European Commission Authorizes COVID-19 Vaccine Moderna in Europe". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ "Moderna becomes third Covid vaccine approved in the UK". BBC News. 8 January 2021. Retrieved 9 February 2022.
- ↑ "Moderna Provides Business Update and Announces Three New Development Programs in Infectious Disease Vaccines". investors.modernatx.com. Retrieved 19 January 2022.
- ↑ "Switzerland Authorizes Moderna's mRNA COVID-19 Vaccine". PharmaNewsIntelligence. 19 January 2021. Retrieved 12 February 2022.
- ↑ "Moderna Hires Amgen Executive As Chief Commercial Officer To Bolster Vaccine Marketing". finance.yahoo.com. Retrieved 9 February 2022.
- ↑ "Moderna poaches Amgen vet as CCO, jump-starting commercial team as it rolls out COVID-19 vaccine". FiercePharma. Retrieved 9 February 2022.
- ↑ "Moderna Appoints Chief Commercial Officer | CHEManager". www.chemanager-online.com. Retrieved 9 February 2022.
- ↑ "Moderna Announces First Participant Dosed in Phase 1/2 Study of Moderna COVID-19 Vaccine in Japan Led by Takeda". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ "Moderna Doses First Patient in Japanese Study of COVID-19 Vaccine". PharmaNewsIntelligence. 28 January 2021. Retrieved 12 February 2022.
- ↑ "Moderna Hires Harvard Stem Cell Researcher Jonathan Hoggatt as Director of Hematology: What You Need to Know". finance.yahoo.com. Retrieved 9 February 2022.
- ↑ "Crain's Headlines: Moderna Partners with Baxter on Vaccine Production". WTTW News. Retrieved 4 February 2022.
- ↑ Schencker, Lisa. "Baxter strikes deal to help Moderna manufacture up to 90 million COVID-19 vaccines this year". chicagotribune.com. Retrieved 12 February 2022.
- ↑ "Baxter, Moderna in deal for COVID vaccine production". Modern Healthcare. 8 March 2021. Retrieved 12 February 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Dose-Ranging Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1283 and mRNA-1273 SARS-CoV-2 Vaccine in Adults Aged 18-55 Years". clinicaltrials.gov. clinicaltrials.gov. 5 January 2022. Retrieved 19 February 2022.
- ↑ "Phase 1, Open-Label, Randomized Study of the Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine (mRNA-1273.351) in Naïve and Previously Vaccinated Adults". clinicaltrials.gov. clinicaltrials.gov. 10 February 2022. Retrieved 19 February 2022.
- ↑ "A Global, Phase 1/2, Open-Label, Dose Optimization Study to Evaluate the Safety, Pharmacodynamics, and Pharmacokinetics of mRNA-3927 in Participants With Propionic Acidemia". clinicaltrials.gov. clinicaltrials.gov. 3 February 2022. Retrieved 19 February 2022.
- ↑ "A Phase 3b, Open-Label, Safety and Immunogenicity Study of SARS-CoV-2 mRNA-1273 Vaccine in Adult Solid Organ Transplant Recipients and Healthy Controls". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ Mendoza, Red (28 April 2021). "Moderna partners with pharmaceutical firm for vaccine application, distribution". The Manila Times. Retrieved 4 February 2022.
- ↑ "Zuellig Pharma To Supply Moderna Covid-19 Vaccine In Southeast Asia". CodeBlue. 4 May 2021. Retrieved 12 February 2022.
- ↑ "Randomised Controlled Trials to Assess Approved SARS-CoV-2 Vaccines in Immunocompromised Patients: A Master Protocol for the Set-up of a Swiss Cohorts Based Trial Platform". clinicaltrials.gov. clinicaltrials.gov. 18 January 2022. Retrieved 19 February 2022.
- ↑ "Gavi signs agreement with Moderna to secure doses on behalf of COVAX Facility". www.gavi.org. Retrieved 12 February 2022.
- ↑ "A Phase 2/3 Study to Evaluate the Immunogenicity and Safety of mRNA Vaccine Boosters for SARS-CoV-2 Variants". clinicaltrials.gov. clinicaltrials.gov. 25 October 2021. Retrieved 19 February 2022.
- ↑ "Moderna partners with Thermo Fisher to scale up COVID-19 vaccine production". Reuters. 1 June 2021. Retrieved 4 February 2022.
- ↑ "Moderna partners with Thermo Fisher to scale up Covid vaccine production". cnbc.com. Retrieved 4 February 2022.
- ↑ "Moderna Partners with UNICEF, Lonza, Thermo Fisher for COVID-19 Vaccine". DCAT Value Chain Insights. 3 June 2021. Retrieved 4 February 2022.
- ↑ "A Phase 2, Randomized, Observer-Blind, Placebo-Controlled, Dose Confirmation Study to Evaluate the Safety, Tolerability, and Immunogenicity of Zika Vaccine mRNA-1893 in Adults Aged 18 Through 65 Years and Living in Endemic and Non-Endemic Flavivirus Areas". clinicaltrials.gov. clinicaltrials.gov. 18 January 2022. Retrieved 19 February 2022.
- ↑ "Moderna appoints J&J veteran Paul Burton as new CMO". Ardent Executive Search | Life Science & Healthcare Recruitment. Retrieved 9 February 2022.
- ↑ "A Phase 1/2, Randomized, Stratified, Observer-Blind, Dose-Ranging Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1010 Seasonal Influenza Vaccine in Healthy Adults 18 Years and Older". clinicaltrials.gov. clinicaltrials.gov. 17 December 2021. Retrieved 19 February 2022.
- ↑ "Post-Marketing Safety of SARS-CoV-2 mRNA-1273 Vaccine in the US: Active Surveillance, Signal Refinement and Self-Controlled Risk Interval (SCRI) Signal Evaluation in HealthVerity". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 19 February 2022.
- ↑ "Kate Cronin to Join Moderna as Chief Brand Officer on July 12". PharmiWeb.jobs. Retrieved 9 February 2022.
- ↑ "Moderna appoints Kate Cronin as Chief Brand Officer". www.adgully.com. Retrieved 9 February 2022.
- ↑ "Ogilvy Health CEO, Kate Cronin, Departs to Take Up Chief Brand Officer Role at Moderna". Branding in Asia Magazine. 30 June 2021. Retrieved 9 February 2022.
- ↑ "Bloomberg - Are you a robot?". www.bloomberg.com. Retrieved 1 April 2022.
- ↑ "A Phase 2, Non-Randomized, Long-Term Extension Study to Evaluate the Immunogenicity and Safety of Cytomegalovirus mRNA-1647 Vaccine in Participants Who Completed Study mRNA-1647-P202". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna Partners with Takeda and the Government of Japan to Supply Additional 50 Million Doses of Moderna's COVID-19 Vaccine and Variant Booster Vaccine Candidate in 2022". www.businesswire.com. 20 July 2021. Retrieved 4 February 2022.
- ↑ "A Phase 1, Dose-Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of a Single Administration of mRNA-6231, Encoding for a Human Serum Albumin - Interleukin-2 Mutein Fusion Protein (HSA-IL2m), in Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 19 February 2022.
- ↑ "A Phase 1, Dose-Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of a Single Administration of mRNA-6231, Encoding for a Human Serum Albumin - Interleukin-2 Mutein Fusion Protein (HSA-IL2m), in Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 16 December 2021. Retrieved 12 February 2022.
- ↑ "Moderna doses first subject in Phase I trial of IL-2 mRNA treatment". Clinical Trials Arena. 3 August 2021. Retrieved 12 February 2022.
- ↑ "Moderna Doses First Patient in Study of Autoimmune mRNA Candidate". PharmaNewsIntelligence. 10 August 2021. Retrieved 12 February 2022.
- ↑ "A Global, Phase 1/2, Open-Label, Dose Optimization Study to Evaluate the Safety, Tolerability, Pharmacodynamics, and Pharmacokinetics of mRNA-3705 in Participants With Isolated Methylmalonic Acidemia Due to Methylmalonyl-CoA Mutase Deficiency". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna announces first patient dosed in mRNA-3705 study". European Pharmaceutical Review. Retrieved 28 March 2022.
- ↑ "Moderna partners with Canadian government on a vaccine plant". cen.acs.org. Retrieved 4 February 2022.
- ↑ "Moderna to build mRNA vaccine production plant in Canada". Pharmaceutical Technology. 11 August 2021. Retrieved 12 February 2022.
- ↑ "Moderna Launches Trial of mRNA Vaccine for Methylmalonic Acidemia". Biopharma from Technology Networks. Retrieved 10 February 2022.
- ↑ "Moderna partners with MLB, NFL teams on vaccine push". www.smartbrief.com. Retrieved 4 February 2022.
- ↑ "Moderna's 'Make it Yours' vaccine campaign taps local partners like Seattle Seahawks, Boston Red Sox". FiercePharma. Retrieved 12 February 2022.
- ↑ "Moderna mRNA-1273 Observational Pregnancy Outcome Study". clinicaltrials.gov. clinicaltrials.gov. 18 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna and ILCM to develop Crigler-Najjar syndrome therapeutic". European Pharmaceutical Review. Retrieved 19 January 2022.
- ↑ "Moderna partners with ILCM to develop medicine for CN-1". NS Healthcare. 8 September 2021. Retrieved 4 February 2022.
- ↑ "Moderna to develop mRNA therapeutic for very rare disease". biopharma-reporter.com. Retrieved 9 February 2022.
- ↑ 234.0 234.1 "mRNA 3745 - AdisInsight". adisinsight.springer.com. Retrieved 13 February 2022.
- ↑ "Immunogenicity of a Third Dose of mRNA-1273 Vaccine (Moderna) Among Cancer Patients". clinicaltrials.gov. clinicaltrials.gov. 25 October 2021. Retrieved 19 February 2022.
- ↑ "Moderna Partners With Carnegie Mellon University To Open AI Academy". thehealthcaretechnologyreport.com. Retrieved 4 February 2022.
- ↑ "Bloomberg - Are you a robot?". www.bloomberg.com. Retrieved 13 February 2022.
- ↑ "First COVID, now HIV — Moderna and La Jolla researchers race to make a vaccine". La Jolla Light. 10 October 2021. Retrieved 19 January 2022.
- ↑ "Moderna initiates Phase III trial of cytomegalovirus vaccine candidate". Clinical Trials Arena. 27 October 2021. Retrieved 9 February 2022.
- ↑ "A Phase 3, Randomized, Observer-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety, and Immunogenicity of mRNA-1647 Cytomegalovirus (CMV) Vaccine in Healthy Participants 16 to 40 Years of Age". clinicaltrials.gov. clinicaltrials.gov. 10 February 2022. Retrieved 19 February 2022.
- ↑ "Moderna partners with gene-editing company Metagenomi". cen.acs.org. Retrieved 4 February 2022.
- ↑ "Metagenomi and Moderna Establish Collaboration to Develop Next-Generation In Vivo Gene Editing Therapeutics". Metagenomi. Retrieved 13 February 2022.
- ↑ "Moderna finally cracks into gene editing with Metagenomi pact thanks to 'irresistible' data". FierceBiotech. Retrieved 13 February 2022.
- ↑ "Moderna scraps lead mRNA chikungunya candidate after phase 1, slowing push beyond prophylactic vaccines". FierceBiotech. Retrieved 9 February 2022.
- ↑ "Moderna Reports Third Quarter Fiscal Year 2021 Financial Results and Provides Business Updates". investors.modernatx.com. Retrieved 12 February 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Placebo-Controlled Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of Cytomegalovirus Vaccine mRNA-1647 When Administered to Healthy Japanese Adults (18-40 Years of Age) in the United States". clinicaltrials.gov. clinicaltrials.gov. 25 January 2022. Retrieved 19 February 2022.
- ↑ "mRNA 3927 - AdisInsight". adisinsight.springer.com. Retrieved 12 February 2022.
- ↑ "Moderna Announces Presentation of Interim Data from Phase 1 Study of mRNA Triplet Program at 2021 SITC Annual Meeting". www.businesswire.com. 12 November 2021. Retrieved 29 March 2022.
- ↑ "A Phase 1, Randomized, First-in-human, Open-label Study to Evaluate the Safety and Immunogenicity of eOD-GT8 60mer mRNA Vaccine (mRNA-1644) and Core-g28v2 60mer mRNA Vaccine (mRNA-1644v2-Core) in HIV-1 Uninfected Adults in Good General Health". clinicaltrials.gov. clinicaltrials.gov. 16 February 2022. Retrieved 19 February 2022.
- ↑ Ellis, Ralph. "Moderna Launches Clinical Trials for HIV Vaccine". WebMD. Retrieved 10 February 2022.
- ↑ Kekatos, Mary (28 January 2022). "Moderna launches clinical trial for HIV vaccine that uses mRNA technology used in COVID shot". ABC7 Los Angeles. Retrieved 11 February 2022.
- ↑ CNN, Anokhi Saklecha. "First patients vaccinated in clinical trial of HIV vaccine that uses Moderna's mRNA technology". CNN. Retrieved 3 February 2022.
- ↑ "Moderna Begins mRNA HIV Vaccine Clinical Trial". Healthline. 1 February 2022. Retrieved 4 February 2022.
- ↑ "AstraZeneca, Moderna's AZD8601 meets primary endpoint in EPICCURE". NS Healthcare. 16 November 2021. Retrieved 17 February 2022.
- ↑ "A Phase 2/3, Randomized, Observer-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of mRNA-1345, an mRNA Vaccine Targeting Respiratory Syncytial Virus (RSV), in Adults ≥60 Years of Age". clinicaltrials.gov. clinicaltrials.gov. 23 December 2021. Retrieved 19 February 2022.
- ↑ "Moderna doses first subject in respiratory syncytial virus vaccine trial". Clinical Trials Arena. 19 November 2021. Retrieved 12 February 2022.
- ↑ "Gavi and Moderna reach agreement for additional supply to COVAX". www.gavi.org. Retrieved 12 February 2022.
- ↑ "Moderna Releases New Vaccine Data Concerning Omicron Variant". PharmTech. Retrieved 18 January 2022.
- ↑ "A Phase 1, Randomized, Observer-Blind, Placebo-Controlled, Dose-Ranging Study of an Epstein-Barr Virus (EBV) Candidate Vaccine, mRNA-1189, in 18- to 30-Year-Old Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 21 January 2022. Retrieved 19 February 2022.
- ↑ Nathan-Kazis, Josh. "Moderna Shipped 800 Million Covid-19 Vaccine Doses in 2021". www.barrons.com. Retrieved 18 January 2022.
- ↑ Kimball, Spencer (6 January 2022). "Moderna CEO warns people may need fourth Covid shot as efficacy of boosters likely to decline over time". CNBC. Retrieved 18 January 2022.
- ↑ "Moderna announces initiation of Phase 1 study of its mRNA-1189 EBV vaccine candidate". News-Medical.net. 7 January 2022. Retrieved 19 January 2022.
- ↑ "Moderna Announces First Participant Dosed in Phase 1 Study of its mRNA Epstein-Barr Virus (EBV) Vaccine". BioSpace. Retrieved 19 January 2022.
- ↑ "Carisma Therapeutics to get $80M in cash and investment through new deal with Moderna". www.bizjournals.com. Retrieved 19 January 2022.
- ↑ Inc, Carisma Therapeutics. "Moderna and Carisma Establish Collaboration to Develop in vivo Engineered Chimeric Antigen Receptor Monocytes (CAR-M) for Oncology". www.prnewswire.com. Retrieved 14 February 2022.
- ↑ "Moderna, Carisma Partner to Develop In Vivo Cancer Therapies". PharmaNewsIntelligence. 24 January 2022. Retrieved 14 February 2022.
- ↑ "Moderna Announces Advances Across Its Industry-Leading mRNA Pipeline and Provides Business Update". finance.yahoo.com. Retrieved 4 February 2022.
- ↑ "Moderna announces combined COVID-19 and flu shot is in the works". WesternMassNews.com. Retrieved 4 February 2022.
- ↑ "Moderna Begins Testing Booster Shot Aimed at Omicron". The Mountaineer. Retrieved 4 February 2022.
- ↑ "An Open-Label Dose-Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of mRNA-3745 in Participants With Glycogen Storage Disease Type 1a (GSD1a)". clinicaltrials.gov. clinicaltrials.gov. 24 January 2022. Retrieved 19 February 2022.
- ↑ Dunleavy, Kevin (8 March 2022). "Moderna will provide access to mRNA tech, but high-income countries will have to pay". Fierce Pharma. Retrieved 28 March 2022.
- ↑ "Queensland researchers partner with Moderna to create fast-tracked, potentially 'game-changing' vaccines". ABC News. 10 March 2022. Retrieved 28 March 2022.
- ↑ "Moderna Announces New Vaccination Programs During its Vaccines Day". Contagion Live. Retrieved 28 March 2022.
- ↑ "McGill University: McGill University joins Moderna in battle against global health threats". India Education | Latest Education News | Global Educational News | Recent Educational News. 26 March 2022. Retrieved 28 March 2022.
- ↑ "McGill joins Moderna's mRNA Access program | Academica Group - Research and Consulting for Higher Education". www.academica.ca. Retrieved 30 March 2022.
- ↑ "Moderna". Google Trends. Retrieved 14 January 2022.
- ↑ "Moderna". www.google.com. Retrieved 14 January 2022.
- ↑ "Moderna". wikipediaviews.org. Retrieved 14 January 2022.