Timeline of BioNTech
This is a timeline of BioNTech, a German biotechnology company focused on immunotherapy.[1] BioNTech is the first company to bring an mRNA-based personalized cancer vaccine to the clinic.[2]
Sample questions
The following are some interesting questions that can be answered by reading this timeline:
- Which diseases and conditions are target of BioNTech's drug pipeline?
- Sort the full timeline by "Disease/condition (when applicable)"
- For the names of their respective candidate drugs, sort the full timeline by "Product research name (when applicable)".
- What are some notable research studies related to BioNTech?
- You will mostly see research papers related by affiliation
- What are the multiple clinical trials conducted by BioNTech?
- Sort the full timeline by "Event type" and look for the group of rows with value "Clinical trial".
- You will mostly see trial launches, but also some sample or notable clinical trial results.
- What are some notable collaborations started between BioNTech and other organizations?
- Sort the full timeline by "Event type" and look for the group of rows with value "Partnership".
- Who are some notable people having been related to BioNTech over the years?
- Sort the full timeline by "Event type" and look for the group of rows with value "Team".
- What are some events describing BioNTech supply of drugs for massive administration?
- Sort the full timeline by "Event type" and look for the group of rows with value "Supply".
- You will see contracts of Pfizer–BioNTech COVID-19 vaccine supply by national governments.
- Other events are described under the following types: "Company launch", "Facility", "Financial", "Side effect", "Strategy", and "Team".
Big picture
| Time period | Development summary | Diseases of focus | More details |
|---|---|---|---|
| 2008-2012 | Early period | Cancer | BioNTech is founded. Mostly research is done in this period. Almost all the research is related to cancer. |
| 2012 onwards | RNA period | Cancer | BioNTech conducts its first trial using naked RNA in 2012, starting a period in that direction.[3] |
| 2020 onwards | COVID-19 period | COVID-19 | BioNTech comes to the forefront after releasing the Pfizer–BioNTech COVID-19 vaccine jointly with Pfizer. |
Summary per year
| Year | Development summary |
|---|---|
| 2008 | BioNTech is founded. |
| 2009 | BioNTech acquires EUFETS and JPT Peptide Technologies.[4][5] |
| 2012 | BioNTech conducts its first trial using naked RNA, in which the molecule is injected directly into lymph nodes.[6] |
| 2013 | BioNTech becomes the first company ever, to start a fully individualized clinical program worldwide.[7] |
| 2014 | BioNTech remains a small company, not even having its own website.[8] |
| 2015 | BioNTech partners with Sanofi to develop up to five cancer immunotherapies, each consisting of a mixture of synthetic mRNAs.[9] |
| 2016 | BioNTech partners with Bayer to develop a new generation of mRNA vaccines and drugs for animal health. In September of the same year, BioNTech partners with Genentech to develop an individualized mRNA tumor vaccine with a specific new etony.[10] |
| 2017 | BioNTech publishes the application of administration of mRNA encoding an antibody as a technology for mRNA-based in vivo delivery of T cell-engaging bispecific antibodies for the potential treatment of cancer.[11] |
| 2018 | BioNTech signs a US$425 million deal with Pfizer to develop an mRNA vaccine for influenza.[12] |
| 2019 | BioNTech announces a US$55 million partnership with the Bill and Melinda Gates Foundation to develop new treatments for HIV/AIDS and tuberculosis.[13] In this year, the company remains an early-stage biotech firmly focused on cancer vaccines.[14] |
| 2020 | BioNTech is catapulted onto the world-stage with the launch of the Pfizer–BioNTech COVID-19 vaccine.[14] The company earns €482.3 million in the year.[15] |
| 2021 | BioNTech earns €18.9 billion in revenue alone.[15] |
Full timeline
| Year | Month and date (approximately) | Disease/condition (when applicable) | Drug/product | Event type | Details |
|---|---|---|---|---|---|
| 2008 | Research | Study co-authored by Uğur Şahin and Özlem Türeci concludes that coupling antigens to major histocompatibility complex Class I trafficking signals increases antigen presentation efficiency.[16] | |||
| 2008 | Company launch | BioNTech is founded as a "spin-off"-company[17] of the Johannes Gutenberg University in Mainz by Uğur Şahin, Özlem Türeci, and Christoph Huber,[18][19] with a seed investment of €150 million.[20] Other co-founders include Andreas and Thomas Strüngmann, Michael Motschmann, and Helmut Jeggle.[21] | |||
| 2008 | April 1 | Cancer (lung) | Research | Study co-authored by Uğur Şahin on autoantibodies in lung cancer concludes that measurement of an autoantibody response to one or more tumour-associated antigens in an optimized panel assay may provide a sensitive and specific blood test to aid the early detection of lung cancer.[22] | |
| 2008 | May 1 | Research | Study co-authored by Uğur Şahin and Özlem Türeci identifies protein coding gene MS4A12 (Membrane Spanning 4-Domains A12) as a colon-selective store-operated calcium channel promoting malignant cell processes.[23] | ||
| 2008 | December 1 | Cancer | Research | Study co-authored by Uğur Şahin and Özlem Türeci concludes that claudin protein CLDN18.2 can be qualified as a novel, highly attractive pan-cancer target for the antibody therapy of epithelial tumors.[24] | |
| 2009 | June | Acquisition | BioNTech acquires Eufets from Fresenius Biotech, retaining all facilities, staff and contracts of the former company. Eufets is a Good Manufacturing Practice (GMP) and is Good Laboratory Practice (GLP) services specialist.[17][25][26] | ||
| 2009 | Research | Study co-authored by Uğur Şahin analizing the guaranteed cost control problem of fuzzy time-delay descriptor system obtains sufficient conditions for guaranteed cost controller by using Lyapunov Krasovski functional method.[27] | |||
| 2010 | January 6 | Cancer | Research | Study co-authored by Uğur Şahin and Özlem Türeci summarizes the current status of antigens identified for their use in cancer immunotherapy. The study highlights that cytotoxic T lymphocytes are the protagonists of an effective cytotoxic antitumoural immune response and motivates the search for antigens recognized by CD8+ T lymphocytes.[28] | |
| 2010 | November 2 | Cancer | Research | Study co-authored by Uğur Şahin on intranodal vaccination with naked antigen-encoding RNA finds potent antigen-specific T-cell immunity against tumors.[29] | |
| 2011 | January 15 | Cancer | Antibody | Research | Study co-authored by Uğur Şahin proves that immunization combining virus-like particles as antigen carriers with specific conformational epitopes of a highly selective differentiation antigen may elicit auto-antibodies with high cytocidal and tumoricidal potential. This result favor active immunization with virus-like particle (VLP)-based vaccines, which at the time gain increasing attention.[30] |
| 2012 | January 11 | Cancer | Research | Study co-authored by Uğur Şahin entitled Exploiting the mutanome for tumor vaccination concludes that dramatically reduced costs and time required for genome-wide discovery of cancer-specific mutations opens the door for individualized immunotherapy of cancer patients.[31] | |
| 2012 | June | Cancer (melanoma) | RBL001/RBL002 | Clinical trial | BioNTech starts a clinical first-in-human dose escalation study with the purpose to evaluate the safety and tolerability of intranodal administration of an RNA-based cancer vaccine targeting two tumor-associated antigens in patients with advanced melanoma.[32] |
| 2013 | December | Cancer (melanoma) | IVAC MUTANOME, RBL001/RBL002 | Clinical trial | BioNTech starts Phase one clinical trial with the purpose to evaluate the safety, tolerability and immunogenicity of intra-nodal administration of a personalized vaccination with IVAC MUTANOME vaccine with or without initial treatment with RBL001/RBL002 vaccine in patients with advanced melanoma.[33] |
| 2014 | March 13 | Cancer | Research | Study co-authored by Uğur Şahin entitled Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma concludes that CT26 cells share molecular features with aggressive, undifferentiated, refractory human colorectal carcinoma cells.[34] CT26 is an N-nitroso-N-methylurethane-(NNMU) induced, undifferentiated colon carcinoma cell line, which was cloned to generate the cell line designated CT26.[35] | |
| 2014 | June | Cancer (breast) | In vitro molecular diagnostic test | Research | BioNTech starts a prospective examination of RNA extracted from tumor material of breast cancer patients treated with a neo-adjuvant therapy. The RNA is analyzed for expression of estrogen receptor (ER 1), progesterone receptor (PgR), HER2 and Ki-67 with MammaTyper, an in vitro molecular diagnostic test.[36] |
| 2014 | August | Cancer (breast) | MammaTyper | Clinical trial | BioNTech starts a clinical trial with the purpose to examine tumor material of a randomized number od breast cancer patients.[37] |
| 2014 | August 22 | Cancer | Research | BioNTech affiliated and Uğur Şahin co-authored study on synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap reports on a number of compounds that can potentially serve either as inhibitors of translation in cancer cells or reagents for increasing expression of therapeutic proteins in vivo from exogenous mRNAs.[38] | |
| 2014 | September 22 | Engineered cell therapy | Research | Study co-authored by Uğur Şahin and Özlem Türeci reports on an approach enabling the rational development of immunotherapy strategies by providing antigen-specific T-cell receptors (TCR) and immunogenic epitopes.[39] | |
| 2014 | October 2 | Facility | BioNTech and Ganymed Pharmaceuticals announce the joint- opening of a new headquarters and research facility of approximatively 10,000 square-meters in Mainz. Scientists from both companies are expected to work in a large lab on a range of pre-clinical and clinical programs mainly in the field of cancer therapies.[7][40] | ||
| 2014 | October | Cancer (glioblastoma) | APVAC1 vaccine plus Poly-ICLC and GM-CSF, APVAC2 vaccine plus Poly-ICLC and GM-CSF | Clinical trial | BioNTech starts a clinical trial with the purpose to "assess the safety and tolerability, feasibility and biological activity (immunogenicity) of the actively personalized vaccination (APVAC) concept in newly diagnosed glioblastoma (GB) patients".[41] |
| 2015 | March 19 | Engineered cell therapy | Research | Study co-authored by Uğur Şahin describes the development of a highly versatile platform for the systematic retrieval of T-cell receptors (TCRs) from single-antigen-reactive T cells and for characterization of their function and specificity. This approach sets the stage for actively personalized immunotherapeutic strategies.[42] | |
| 2015 | March | Cancer (melanoma) | Lipo-MERIT | Clinical trial | BioNTech starts a clinical trial to evaluate a tetravalent RNA-lipoplex cancer vaccine targeting four tumor-associated antigens in patients with advanced melanoma.[43] |
| 2015 | November 26 | Research | Study co-authored by Uğur Şahin explores reprogramming of human fibroblasts and Bblood-derived endothelial progenitor cells using nonmodified RNA for reprogramming and immune evasion. The study presents novel data demonstrating integration-free RNA reprogramming via co-transfection of synthetic non-modified reprogramming and vaccinia virus immune evasion mRNAs coding E3, K3 and B18R (EKB).[44] | ||
| 2016 | January | Cancer (pancreatic) | MVT-5873 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate HuMab-5B1 (MVT-5873) in subjects with pancreatic cancer or other cancer antigen 19-9 (CA19-9) positive malignancies.[45] |
| 2016 | April 22 | Cancer | Research | Study co-authored by Uğur Şahin and Özlem Türeci on mutant major histocompatibility complex (MHC) class II epitopes concludes that these drive therapeutic immune responses to cancer in mice.[46] | |
| 2016 | May 10 | Partnership | BioNTech partners with Bayer to develop novel, first-in-class mRNA vaccines and therapeutics specifically for animal health applications. BioNTech agrees to contribute its proprietary mRNA technology platform, formulation development capabilities, immunology expertise and intellectual property; while Bayer's Animal Health unit agrees to provide its development expertise and disease know-how. The collaboration is expected to benefit BioNTech's extensive human health program.[47] | ||
| 2016 | June 16 | Research | Study co-authored by Uğur Şahin and Özlem Türeci shows that RNA-LPX encoding viral or mutant neo-antigens or endogenous self-antigens induce strong effector and memory T-cell responses, and mediate potent IFNα-dependent rejection of progressive tumours.[48] | ||
| 2016 | July 11 | Cancer (pancreatic) | MVT-2163, MVT-5873 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate MVT-2163 and MVT-5873 used in performing PET scans. The study is designed to determine the best time and dose of these agents that result in the best PET image of a tumor.[49] |
| 2016 | September 21 | Cancer | Partnership | BioNTech partners with American biotechnology corporation Genentech with the purpose to develop, manufacture and commercialize novel messenger RNA (mRNA)-based, individualized cancer vaccines. The collaboration aims to combine Genentech's cancer immunotherapy portfolio and research program with BioNTech's proprietary mRNA cancer vaccine technology platform, and personalized medicine expertise.[50][51] | |
| 2016 | October 11 | Research | Study co-authored by Uğur Şahin describes the steps for translating novel RNA nanomedicines into clinical trials. The former include second generation of RNA vaccines encompassing intravenously injectable RNA nanoparticle formulations (lipoplexes), made up from lipid excipients, denoted RNA(LIP).[52] | ||
| 2016 | October | Cancer (breast) | IVAC_W_bre1_uID, IVAC_M_uID | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the RNA-Immunotherapy of IVAC investigational medicinal products (IMPs) IVAC_W_bre1_uID and IVAC_M_uID.[53] IVAC_W_bre1_uID (Antigen-targeted Personalized Breast Cancer Vaccine) is an individualized, therapeutic cancer vaccine composed of liposomes containing RNA encoding two or three tumor associated antigens that are specifically expressed in the patient's individual cancer selected "off the shelf" and p53 RNA, with potential immunostimulatory and antineoplastic activities.[54] IVAC_M_uID is an on-demand manufactured Individualized NeoAntigen Specific Immunotherapy (iNeST) encoding neoepitopes derived from up to 20 cancer mutations determined by next-generation sequencing.[55] |
| 2016 | October | Cancer (bladder) | NEO-PV-01 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate if the treatment with NEO-PV-01 + adjuvant in combination with nivolumab is safe and useful for patients with certain types of cancer. The study also aims to investigate if NEO-PV-01 + adjuvant with nivolumab may represent a substantial improvement over other available therapies such as nivolumab alone.[56] NEO-PV-01 is a personalised neoantigen-based vaccine in development by BioNTech for the treatment of cancer.[57] |
| 2017 | April 11 | HPV | HPV vaccine | Clinical trial | BioNTech starts a phase I/II vaccine dose escalation study with two different arms, one aimed to perform intrapatient dose escalation in patients with previously treated HPV16+ Head & Neck Cancer using two dose cohorts to establish a safe, tolerable and recommended dose of HPV vaccine, and a second aimed to perform intrapatient dose escalation in patients with advanced HPV16+ cancer (head and neck, anogenital, penile or cervical) using a single cohort to establish a safe, tolerable and recommended dose of HPV vaccine.[58] |
| 2017 | March 9 | Zika virus | Publication | BioNTech-affiliated study provides data demonstrating that lipid-nanoparticle-encapsulated nucleoside-modified mRNA elicits rapid and durable protective immunity and represents a new and promising vaccine candidate against Zika virus[59] | |
| 2017 | June 1 | Cancer (pancreatic) | MVT-1075m, MVT-5873 | Clinical trial | BioNTech starts a clinical trial to evaluate MVT-5873 in combination with human monoclonal antibody 5B1 (MVT-1075) in subjects with previously treated, CA19-9 positive malignancies (e.g., pancreatic adenocarcinoma).[60] MVT-5873 is designed to kill cancer by targeting CA19-9, a protein on the surface of these pancreatic cancer cells.[61] |
| 2017 | July 20 | Cancer | Publication | Researchers at BioNTech publish study titled Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer, which demonstrates that individual mutations can be exploited, thereby opening a path to personalized immunotherapy for patients with cancer.[62] | |
| 2017 | July 23 | Publication | BioNTech affiliated researchers publish study proposing that the manufacture of pharmaceutical mRNA, for being fast, could accelerate the clinical development of novel bispecific antibodies.[63] | ||
| 2017 | September 6 | Partnership | BioNTech announces having entered into a broad and comprehensive non-exclusive patent licensing agreement with pharmaceutical company CELLSCRIPT covering nucleoside-modified messenger RNA (mRNA) for use in all in vivo applications, including therapeutic and non-therapeutic applications in humans and animals.[64] | ||
| 2017 | December 21 | Cancer | Autogene cevumeran, atezolizumab | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate autogene cevumeran (RO7198457) as a single agent and in combination with atezolizumab in participants with locally advanced or metastatic tumors.[65] |
| 2018 | January 4 | Funding | BioNTech announces having completed a US$270 million Series A round of financing, led by the Redmile Group and joined by Janus Henderson Investors, Invus, Fidelity Management & Research Company and several European family offices in addition to The Struengmann Family Office, an existing investor in BioNTech. The company is expected to use the capital to further advance its clinical pipeline of individualized immunotherapies covering a number of new approaches including mRNA and CAR-T / T-Cell receptors for the treatment of cancer and other diseases with high medical need.[66] | ||
| 2018 | April 13 | Antibody | Research | Study co-authored by Uğur Şahin and Özlem Türeci confirms the genetically simply transferable C-terminal polyhistidine-peptide as a general stabilizing element for chimeric HBcAg virus-like-particles to increase their suitability.[67] Hepatitis B viral protein HBcAg is a crucial serology marker of hepatitis B infection and patient follow-up.[68] | |
| 2018 | May 4 | Cancer (lung) | NEO-PV-01, pembrolizumab | Clinical trial | BioNTech starts a clinical trial to evaluate if treatment with NEO-PV-01 in combination with pembrolizumab and chemotherapy (pembrolizumab/chemotherapy) is safe and useful for patients with lung cancer. The study also aims to assess if the NEO-PV-01 vaccine, when given together with pembrolizumab and chemotherapy, can improve your response compared with pembrolizumab and chemotherapy treatment alone.[69] NEO-PV-01 is a personal neoantigen vaccine of up to 20 peptides (14-35 amino acids) designed based on bioinformatic analysis of a patient’s neoantigen and human leukocyte antigen profile.[70] |
| 2018 | May 29 | Team | Özlem Türeci is appointed by BioNTech as Chief Medical Officer.[71] | ||
| 2018 | July 10 | Partnership | BioNTech partners with Genevant Sciences partner to develop five mRNA therapeutic programs for rare diseases with high unmet medical need. The companies also agree a series of exclusive licenses covering the application of Genevant’s delivery technology to five of BioNTech’s oncology programs.[72] | ||
| 2018 | August 16 | Partnership | BioNTech signs a deal with Pfizer with the purpose to develop mRNA influenza vaccines. Under the deal, Pfizer agrees to provide US$120 million in upfront and near-term payments to enter into the research and development collaboration. BioNTech is also eligible additional $305m million in potential development, regulatory and commercial milestones, as well as receiving tiered royalties on worldwide sales after the program reaches commercialization.[73] "BioNTech signs $425m flu vaccine deal with Pfizer"[74] | ||
| 2018 | September 1 | Partnership | BioNTech partners with biopharmaceutical company Scancell, with the purpose to develop T-cell receptor (TCR)-based therapies. The collaboration aims to combine Scancell’s Moditope immunotherapy platform and BioNTech’s high-throughput platform for the cloning and characterization of T cell receptors.[75] | ||
| 2018 | October 8 | Cancer (melanoma) | NEO-PV-01 | Clinical trial | BioNTech starts a clinical trial with the purpose to demonstrate that the NEO-PV-01 vaccine, either with APX005M or ipilimumab, and nivolumab is safe for the treatment of patients with advanced or metastatic melanoma.[76] |
| 2018 | November 5 | Partnership | BioNTech partners with the University of Pennsylvania to develop nucleoside-modified mRNA vaccine. The task is appointed to Drew Weissmann’s UPenn lab with taking vaccines against up to 10 infectious diseases through to the completion of IND-enabling studies.[77] | ||
| 2018 | November 28 | Cancer (breast) | Clinical trial (result) | BioNTech announces new results from a clinical performance evaluation study of the CE-marked IVD MammaTyper. For this study, samples from the trial cohorts were used to validate a pre-defined score and cut-off integrating highly standardized mRNA measurements of genes ERBB2 (HER2), ESR1 (ER), PGR (PR) and MKI67 (Ki-67) by in vitro diagnostic test MammaTyper. The score allows the prediction of pathological complete response after neoadjuvant chemotherapy based on the pre-treatment biopsy. The cut-off accurately separates patients with a good response rate from patients with a low probability of pCR for which a different treatment than NACT or additional post-neoadjuvant treatment may be considered. Results also demonstrate that standardized measurement of ERBB2, ESR1, PGR and MKI67 mRNA is comparable to the determination of the markers on immunohistochemistry level and is strongly associated with response to neoadjuvant chemotherapy as well as long-term outcome.[78] | |
| 2019 | January 3 | Cancer (metastatic neoplasm) | SAR441000 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate intratumoral administration of SAR441000 as monotherapy and in combination with cemiplimab in patients with advanced solid tumors.[79] SAR441000 is a novel saline-formulated mixture of four mRNAs encoding interleukin-12 single chain, interferon alpha-2b, granulocyte-macrophage colony-stimulating factor, and interleukin-15 sushi that were identified as mediators of tumor regression across different murine tumor models.[80] The candidate belongs to BioNTech's Intratumoral Immunotherapy platform.[81] |
| 2019 | January 4 | Funding | French multinational Sanofi agrees to invest €80 million (US$91 million) in BioNTech and extend its cancer collaboration with the company, as BioNTech and Sanofi prepare to start clinical development of the first cancer immunotherapy to emerge from their 2015 pact.[82] "Sanofi Invests €80M in mRNA Cancer Vaccine Company"[83][84] | ||
| 2019 | January 4 | Strategy | BioNTech announces that the company’s shareholders have decided to convert BioNTech’s corporate legal entity from the German “AG” (Aktiengesellschaft or share-based corporation) to a European company (Societas Europaea or “SE”). This change is considered a logical step in the development of BioNTech's worldwide operations, as the company becomes a public limited company under EU law, gaining a supranational aspect. The transition enables greater flexibility to operate globally and engage in fund-raising transactions and partnerships with other international corporations.[85] | ||
| 2019 | January 8 | Cancer (melanoma) | RO7198457, pembrolizumab | Clinical trial | BioNTech starts a clinical trial to evaluate RO7198457 plus pembrolizumab compared with pembrolizumab alone in patients with previously untreated advanced melanoma.[86] RO7198457 is a systemically administered RNA-Lipoplex iNeST designed to stimulate T cell responses against neoantigens.[87] |
| 2019 | January 23 | Acquisition | BioNTech announces agreement with Mab Discovery GmbH to acquire its operational antibody generation unit.[88][89][90] | ||
| 2019 | April 19 | Small Molecule Immunomodulator | Research | Study co-authored by Uğur Şahin and Özlem Türeci finds that systemic administration of synthetic agonist SC1 mobilizes innate and adaptive immunity and is highly potent as single agent in mice and thereby provides a rationale for clinical testing of this compound.[91] A small molecule agonist of protein TLR7, SC1 demonstrates to stimulate natural killer cells and therefore mediates efficient immune responses, and shows an effective anti-metastatic activity in vivo.[92] | |
| 2019 | May 8 | Cancer | Acquisition | BioNTech purchases MabVax Therapeutics' cancer drug assets alongside much of its infrastructure and labs with the purpose to build up a new research hub in San Diego and spread its geographic reach.[93][94] | |
| 2019 | May 14 | Cancer | GEN1046 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the safety of GEN1046 as monotherapy and in combination therapies in patients with malignant solid tumors.[95] GEN1046 is a proprietary bispecific antibody that was developed using DuoBody technology[96], a versatile platform technology for the discovery and development of bispecific antibodies that may improve antibody therapy of cancer.[97] |
| 2019 | June 17 | DuoBody-PD-L1x4-1BB | Clinical trial | BioNTech starts the first-in-human Phase I/IIa study with DuoBody-PD-L1x4-1BB, the latter being a bispecific antibody in joint development with Genmab A/S, which is studied in patients with metastatic or unresectable malignant solid tumors, who are not candidates for standard therapy. [98] | |
| 2019 | June 23 | Acquisition | BioNTech announces acquisition of MAB Discovery’s operational antibody generation unit. Under the terms of the agreement, BioNTech would acquire all assets, employees and proprietary know-how for mAb generation from MAB Discovery. MAB Discovery would retain ownership of and all rights to both its proprietary preclinical development pipeline and its existing third-party service agreements.[99] | ||
| 2019 | July 9 | Cancer | Funding | BioNTech raises a Series B of €289.5 million (US$325M) to develop individualized cancer vaccines. The company aims to use the money to fuel the clinical development of its lead program consisting in an individualized mRNA-based immunotherapy for treating melanoma, which completed a phase I trial in 2017. The funding is also expected to reinforce manufacturing capabilities, as well as other programs in the pipeline, including other mRNA cancer vaccines, CAR T-cell therapies, and small molecules.[100][101] | |
| 2019 | September 4 | HIV/AIDS, tuberculosis | Partnership | BioNTech announces having signed an agreement with the Bill & Melinda Gates Foundation to develop preclinical vaccine and immunotherapy candidates to prevent HIV and tuberculosis infection as well as to lead to durable antiretroviral therapy-free remission of HIV disease. The partnership includes an initial equity investment of US$55 million, with total funding under the collaboration able to reach $100 million through potential future grant funding from the Gates Foundation.[102][103] | |
| 2019 | September 17 | Cancer (solid tumor) | BNT 312 | Clinical trial | BioNTech starts a clinical trial to evaluate the safety and anti-tumor activity of GEN1042 in patients with metastatic or locally advanced solid tumors.[104] GEN 1042 (also known as BNT 312) is an IgG1 Fc-silenced bispecific antibody developed by Genmab and BioNTech, for the treatment of various solid tumors.[105] |
| 2019 | November 25 | Cancer (ovarian) | W_ova1 Vaccine | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate a liposome formulated mRNA vaccine in combination with (neo-)adjuvant chemotherapy against ovarian cancer.[106] |
| 2019 | November | Cancer (prostate) | BNT112, cemiplimab | Partnership | BioNTech signs a clinical trial supply agreement with Regeneron Pharmaceuticals to supply cemiplimab for use in combination with BioNTech’s BNT112 in a first-in-human Phase 1/2 trial in advanced prostate cancer. Regeneron agrees to supply their PD-1 checkpoint inhibitor Libtayo (cemiplimab) at no cost to BioNTech for use in combination with BNT112 in BioNTech’s planned Phase 1/2 trial. Both companies each retain full commercial rights to BNT112 and Libtayo respectively.[107] |
| 2019 | December | Cancer, other diseases | Funding | BioNTech receives a €50 million loan, to finance the development of its patient-specific immunotherapies for the treatment of cancer and other serious diseases, from the European Investment Bank (EIB) as part of the European Commission Investment Plan for Europe.[108] | |
| 2019 | December 19 | Cancer (prostate | W_pro1 | Clinical trial | BioNTech starts a clinical trial to evaluate W_pro1 cancer vaccine (W_pro1) monotherapy or in combination with cemiplimab in patients with metastatic castration resistant prostate cancer.[109] |
| 2020 | January 2 | Cancer (solid tumors) | CLDN6 | Research | Study co-authored by Uğur Şahin introduces the developmentally regulated tight junction protein claudin 6 (CLDN6) as a chimeric antigen receptor target in solid tumors and a strategy to overcome inefficient CAR-T cell stimulation in vivo. The study demonstrates that a nanoparticulate RNA vaccine, designed for body-wide delivery of the CAR antigen into lymphoid compartments, stimulates adoptively transferred CAR-T cells. Chimeric antigen receptor (CAR)–T cells have shown efficacy in patients with B cell malignancies.[110] |
| 2020 | March 17 | COVID-19 | Partnership | Biontech and Pfizer announce a partnership for COVID-19 vaccine development: Biontech is developing mRNA vaccines for COVID-19, that Pfizer will then produce at scale and distribute.[111][112] | |
| 2020 | March 20 | COVID-19 | Background | The World Health Organization declares coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1, a pandemic.[113] | |
| 2020 | April 23 | COVID-19 | BNT162 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the safety and effects of four different vaccines (BNT162a1, BNT162b1, BNT162b2, and BNT162c2) against COVID-2019 in healthy and immunocompromised adults.[114] |
| 2020 | April 29 | COVID-19 | BNT162b1, BNT162b2 | Clinical trial | BioNTech starts a clinical trial to evaluate the safety, tolerability, and immunogenicity of 3 different SARS-CoV-2 RNA vaccine candidates against COVID-19 and the efficacy of 1 candidate.[115] |
| 2020 | June 19 | Cancer | BNT411 | Clinical trial | BioNTech starts a first-in-human (FIH) trial aiming to establish a safe dose of BNT411 as a monotherapy and in combination with atezolizumab, carboplatin and etoposide. "Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy Trial of BNT411"[116] Developed by BioNTech, BNT411 is a small molecule toll-like receptor 7 (TLR7) agonist for the treatment of solid tumors.[117] |
| 2020 | July 20 | COVID-19 | BNT162b1 | Supply | The Government of the United Kingdom secures 30 million doses of Pfizer–BioNTech COVID-19 vaccine.[118] |
| 2020 | July 27 | Financial | BioNTech announces the closing of its previously announced underwritten offering of 5,500,000 American Depositary Shares, each representing one of its ordinary shares, at a public offering price of $93.00 per ADS, for gross proceeds of approximately $512 million, before deducting underwriting discounts and commissions and other offering expenses payable by the company.[119] | ||
| 2020 | July 28 | COVID-19 | BNT162b1 | Clinical trial | BioNTech starts a clinical trial to evaluate the safety and immunogenicity of Pfizer–BioNTech COVID-19 vaccine in Chinese healthy subjects.[120] |
| 2020 | July 29 | Cancer (melanoma, potentially others) | Research | Study co-authored by Uğur Şahin and Özlem Türeci finds that RNA-lipoplex vaccination is a potent immunotherapy in patients with checkpoint-inhibitor-experienced melanoma, and suggests the general utility of non-mutant shared tumour antigens as targets for cancer vaccination.[121] | |
| 2020 | July 31 | COVID-19 | BNT162b1 | Supply | Pfizer and BioNTech agree to supply Japan with 120 million doses of their COVID-19 vaccine in the first half of 2021.[122] |
| 2020 | August 5 | COVID-19 | BNT162b1 | Supply | Pfizer and BioNTech announce an agreement with the Government of Canada to supply their Pfizer–BioNTech COVID-19 vaccine, subject to clinical success and Health Canada approval.[123] |
| 2020 | September 9 | COVID-19 | BNT162b3 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the safety and effects of one BNT162 vaccine against COVID-19 in healthy adults.[124] |
| 2020 | September 16 | Cancer (solid tumor) | CLDN6 CAR-T, CLDN6 RNA-LPX | Clinical trial | BioNTech starts a clinical trial to evaluate the safety and efficacy of CLDN6 CAR-T and CLDN6 RNA-LPX claudins.[125] CLDN6 protein is a tumor-specific antigen expressed in solid tumors but silenced in adult healthy tissue during organogenesis, leading to the development of a second-generation chimeric antigen receptor T-cell product with high affinity and specificity for CLDN6.[126] |
| 2020 | September | COVID-19 | BNT162b1 | Supply | The Government of Germany signs a private deal with Pfizer and BioNTech for 30 million extra doses of their vaccine. The deal is arranged outside the collective EU purchasing scheme.[127] |
| 2020 | September 30 | COVID-19 | BNT162b1 | Clinical trial (result) | A paper co-authored by Uğur Şahin and Özlem Türeci reports safety, tolerability and antibody response data from an ongoing placebo-controlled, observer-blinded phase I/II COVID-19 vaccine trial with BNT162b1, a lipid nanoparticle-formulated nucleoside-modified mRNA that encodes the receptor binding domain (RBD) of the SARS-CoV-2 spike protein. The vaccine is reported to elicit human antibody and T helper type 1 T cell responses.[128] |
| 2020 | October 14 | COVID-19 | NT162b1, BNT162b2 | Research | A study on safety and immunogenicity of BioNTech RNA-Based Covid-19 vaccine candidates BNT162b1 and BNT162b2, reports that BNT162b2 is associated with a lower incidence and severity of systemic reactions than BNT162b1, particularly in older adults. Also, "in both younger and older adults, the two vaccine candidates elicited similar dose-dependent SARS-CoV-2–neutralizing geometric mean titers, which were similar to or higher than the geometric mean titer of a panel of SARS-CoV-2 convalescent serum samples".[129] |
| 2020 | October 21 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the safety, tolerability, and immunogenicity of an RNA COVID-19 vaccine candidate BNT162b2 in healthy Japanese adults.[130] |
| 2020 | November | COVID-19 | BNT162b2 | Clinical trial | Pfizer–BioNTech COVID-19 vaccine BNT162b2 enters Phase III clinical trials, with over 40,000 people participating.[131] |
| 2020 | November 5 | COVID-19 | BNT162b2 | Supply | The Government of Australia pre-orders 10 million doses of the Pfizer–BioNTech COVID-19 vaccine.[132] |
| 2020 | November 11 | COVID-19 | BNT162b2 | Supply | The European Union pre-orders 300 million doses of the BioNTech-Pfizer vaccine, at an initial price of €12 per dose. Under the agreement, the vaccine-producing pair, BioNTech and Pfizer are subject to a tightened legal obligation to bring all the experience they have gained to the European Medicines Agency for review.[133] |
| 2020 | November 20 | COVID-19 | BNT162b2 | Authorization (application) | BioNTech and Pfizer apply to the U.S. Food and Drug Administration for an emergency use authorization for their COVID-19 vaccine.[134] |
| 2020 | December 1 | Cancer (melanoma) | NEO-PTC-01 | Clinical trial | BioNTech starts a clinical trial with the purpose to investigate the safety and activity of NEO-PTC-01 in patients with unresectable or metastatic melanoma.[135] NEO-PTC-01 is a personal neoantigen-targeted T cell therapy candidate derived from patients’ peripheral blood mononuclear cells and consisting of multiple T cell populations targeting the most therapeutically relevant neoantigens from each patient’s tumor.[136] |
| 2020 | December 2 | COVID-19 | BNT162b2 | Authorization (approval) | Pfizer–BioNTech COVID-19 vaccine becomes the first COVID‑19 vaccine to be authorized by a stringent regulatory authority for emergency use, when the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) gives temporary regulatory approval.[137][138][139][140] It also becomes the first cleared vaccine for regular use.[141] |
| 2020 | December 4 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the safety and immunogenicity of SARS-CoV-2 mRNA Vaccine (BNT162b2) in chinese healthy adults 18-85 years of age.[142] |
| 2020 | December 10 | COVID-19 | BNT162b2 | Research | A study on safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine concludes that a two-dose regimen of BNT162b2 confers 95% protection against Covid-19 in persons 16 years of age or older, while safety over a median of 2 months is found to be similar to that of other viral vaccines.[143] |
| 2020 | December 11 | COVID-19 | BNT162b2 | Authorization (approval) | The United States FDA grants an EUA for the Pfizer–BioNTech COVID‑19 vaccine.[144] |
| 2020 | December 21 | COVID-19 | BNT162b2 | Authorization (approval) | The European Medicines Agency (EMA) authorizes a one-year conditional use of the BioNTech-Pfizer vaccine in the European Union. Emer Cooke, Director of the EMA, states that the benefits of the vaccine far outweigh the potential risks.[145] |
| 2020 | December 23 | COVID-19 | BNT162b2 | Authorization (approval) | Argentina approves the Pfizer–BioNTech COVID-19 vaccine.[146] |
| 2020 | December 26 | COVID-19 | BNT162b2 | Authorization (approval) | Following the authorization, initial mass BioNTech-Pfizer vaccination against Covid-19 commences in Hungary and Slovakia.[147] |
| 2020 | December | COVID-19 | BNT162b2 | Side effect | 1,893,360 first doses of Pfizer–BioNTech COVID‑19 vaccine administration result in 175 cases of severe allergic reaction, of which 21 are anaphylaxis.[148] |
| 2021 | January 7 | Cancer (head and neck) | BNT113 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate mRNA vaccine candidate BNT113 in combination with pembrolizumab versus pembrolizumab alone for patients with a form of head and neck cancer positive for human papillomavirus 16 and expressing the protein PD-L1.[149] |
| 2021 | January 8 | COVID-19 | BNT162b2 | Supply | The European Union orders an additional 200 million doses of BioNTech-Pfizer vaccine, with an option for 100 million more.[150] |
| 2021 | January 8 | Encephalomyelitis | Research | Study co-authored by Uğur Şahin explores a noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. The research team designed a messenger RNA vaccine strategy that lacks adjuvant activity and delivers multiple sclerosis autoantigens into lymphoid dendritic cells.[151] | |
| 2021 | January 26 | Cancer | BNT151 | Clinical trial | BioNTech starts a clinical trial with the purpose to assess product candidate BNT151 as a monotherapy and in combination with other agents in patients with solid tumors.[152] BNT151 belongs to the RiboCytokines (mRNA-encoded cytokines) platform of BioNTech mRNA drug class.[81] |
| 2021 | February 1 | COVID-19 | BNT162b | Research | Study co-authored by Uğur Şahin and Özlem Türeci reports that BNT162b vaccines protect rhesus macaques from SARS-CoV-2.[153] |
| 2021 | February 8 | COVID-19 | BNT162b2 | Research | A study supported by Pfizer and BioNTech evaluates the effect of several key spike mutations from the SARS-CoV-2 Alpha variant and SARS-CoV-2 Omicron variant strains on BNT162b2 vaccine-elicited neutralization. The results indicate small effects of these mutations on neutralization by sera elicited by two BNT162b2 doses.[154] |
| 2021 | February 15 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a Phase 3 clinical trial to evaluate the safety, tolerability, and immunogenicity of multiple production lots and dose levels of BNT162b2 RNA-Based COVID-19 vaccines against COVID-19 in healthy participants.[155] |
| 2021 | February 16 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a Phase 2/3, randomized, placebo-controlled, observer-blind study with the purpose to evaluate the administration of 30 µg of BNT162b2 or placebo administered in 2 doses, 21 days apart, in approximately 700 healthy pregnant women 18 years of age or older vaccinated at 24 to 34 weeks' gestation. [156] |
| 2021 | March 8 | Colorectal cancer | RO7198457 | Clinical trial | BioNTech starts a Phase II clinical trial to compare the efficacy of mRNA-based cancer vaccine candidate RO7198457 versus watchful waiting in patients with circulating tumor DNA (ctDNA) positive, surgically resected Stage II/III rectal cancer, or Stage II (high risk)/Stage III colon cancer.[157] RO7198457 is a systemically administered RNA-Lipoplex iNeST designed to stimulate T cell responses against neoantigens.[158] |
| 2021 | March 24 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a Phase 1/2/3 clinical trial to evaluate RNA vaccine candidate BNT162b2 against COVID-19 in healthy children and young adults.[159] |
| 2021 | March 31 | COVID-19 | BNT162b2 | Clinical trial (result) | Pfizer and BioNTech announce the results of their Pfizer–BioNTech COVID-19 vaccine Phase 3 clinical trial in subjects aged 12 to 15, suggesting the shot is 100 per cent effective in teens. The release says the results “exceeded those recorded earlier in vaccinated participants aged 16 to 25 years old, and was well tolerated.”[160] |
| 2021 | April 1 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate safety, tolerability, and immunogenicity of multiple formulations of BNT162b2 against COVID-19 in healthy adults.[161] |
| 2021 | May 10 | Cancer (pancreatic) | MVT-2163, MVT-5873 | Clinical trial) | BioNTech starts a clinical trial with the purpose to see how well the experimental imaging agent 89Zr-DFO-HuMab-5B1 attaches to pancreatic tumors, and to find out whether PET/CT scans done with this imaging agent produce better images of cancer.[162] 89Zr-DFO-HuMab-5B1 is a radioimmunoconjugate that can be used to delineate urothelial carcinomas by positron emission tomography imaging and may provide tumor-specific information prior to, during, and after systemic therapies.[163] |
| 2021 | May 19 | COVID-19 | BNT162b2 | Side effect | Experts commissioned by the Norwegian Medicines Agency conclude that the Pfizer-BioNTech vaccine is the likely cause of ten deaths of frail elderly patients in Norwegian nursing homes. They say that people with very short life expectancies have little to gain from vaccination, having a real risk of adverse reactions in the last days of life and of dying earlier.[164] |
| 2021 | May 19 | Cancer (melanoma) | BNT111 | Clinical trial | BioNTech starts interventional clinical trial with the purpose to evaluate intravenous therapeutic cancer vaccine candidate BNT111 + cemiplimab in anti-PD-1-refractory/relapsed patients with unresectable Stage III or IV melanoma. [165] BNT111 encodes for a fixed set of four cancer-specific antigens optimized for immunogenicity and delivered as RNA-lipoplex formulation.[166] |
| 2021 | June 8 | Cancer | BNT152, BNT153 | Clinical trial | BioNTech starts an interventional clinical trial with the purpose to evaluate product candidates BNT152 and BNT153 in various solid tumor indications. Both candidates belong to the RiboCytokines (mRNA-encoded cytokines) platform of BioNTech mRNA drug class.[167] |
| 2021 | June 15 | Cancer (solid tumor) | GEN1046 | Clinical trial | BioNTech starts a clinical trial to evaluate proprietary bispecific antibody GEN1046.[168] |
| 2021 | June 25 | COVID-19 | BNT162b2 | Side effect | The US Food and Drug Administration adds a warning about the risk of rare heart inflammation (myocarditis) to the specifications of both BioNTech-Pfizer and Moderna vaccines.[169][170] |
| 2021 | July 1 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a clinical trial to evaluate the safety, tolerability, and efficacy of a booster dose of BNT162b2 when administered to participants in participants since 12 years of age having previously received 2 doses of BNT162b2 at least 6 months prior to randomization.[171] |
| 2021 | July 2 | Cancer (colorectal) | AdCTx | Research | BioNTech starts an epidemiological study with the purpose to monitor circulating tumor DNA (ctDNA) status in participants with Stage II (high risk)/III colorectal cancer following resection/prior to adjuvant chemotherapy (AdCTx), during the course of AdCTx and for a period of 21 months thereafter, according to CRC stages and disease characteristics.[172] |
| 2021 | July 12 | COVID-19 | BNT162b2 | Supply | BioNTech announces a deal to provide Taiwan with 10 million doses of the Pfizer–BioNTech COVID-19 vaccine, with the purpose to end a serious shortage on the island.[173] |
| 2021 | July 15 | COVID-19 | BNT162b2 | Supply | Indonesia's Food and Drug Monitoring Agency (BPOM) grants emergency use authorization (EUA) to the Pfizer-BioNTech COVID-19 vaccine in a deal to supply 50 million doses to the country.[174] |
| 2021 | July 26 | COVID-19 | BNT162b2s01, BNT162-01, BNT162-04 | Clinical trial | BioNTech starts a trial to evaluate the safety and immunogenicity of one or two boosting doses of BNT162b2 or one dose of BNT162b2s01 (Variant of concern (VOC) strain B.1.351) in BNT162-01 trial participants, or two boosting doses of Comirnaty in BNT162-04 trial participants.[175] |
| 2021 | August 18 | Cancer (leukemia) | NeoVax, cyclophosphamide, pembrolizumab | Clinical trial | BioNTech starts a clinical trial with the purpose to study a novel type of vaccine as a possible treatment for chronic lymphocytic leukemia. The interventions involved in the study consist in a personalized neoantigen vaccine, poly-ICLC, cyclophosphamide, and pembrolizumab.[176] |
| 2021 | August 23 | COVID-19 | BNT162b2 | Authorization (approval) | The Pfizer–BioNTech COVID-19 vaccine becomes the first COVID-19 vaccine to be approved in the United States by the Food and Drug Administration for those aged sixteen years and older.[177][178][179] |
| 2021 | August 25 | COVID-19 | BNT162b2 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate the safety and immunogenicity of Pfizer–BioNTech COVID-19 vaccine in healthy subjects.[180] |
| 2021 | September 8 | Cancer | Research | Study co-funded by Sanofi and BioNTech investigating local delivery of mRNA-encoded cytokines finds that it promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Having demonstrated the feasibility of intratumoral mRNA delivery, the study gives path to explore the therapeutic potential of expressing immune-modulatory proteins within the tumor.[181] | |
| 2021 | September 29 | COVID-19 | BNT162b2 | Authorization (application) | Pfizer and BioNTech start submission of data to the US Food and Drug Administration asking for authorization of the Pfizer–BioNTech COVID-19 vaccine for children ages 5- 11 (five to 11), after considering the increased number of infections amongst children, a large number of whom having been infected since the emergence of the SARS-CoV-2 Delta variant.[182] |
| 2021 | October 15 | COVID-19 | BNT162b2 | Clinical trial | BioNTech launches an interventional clinical trial with the purpose to evaluate BNT162b2 in immunocompromised participants two years and older.[183] |
| 2021 | October 27 | Cancer (lung) | GEN1046 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate GEN1046 as a single agent or in combination with another anti-cancer therapy for treatment of recurrent (non-small cell) lung cancer.[184] |
| 2021 | November 10 | COVID-19 | BNT162b2 | Recommendation | Germany's Standing Committee on Vaccination (STIKO) recommends that only the Pfizer–BioNTech COVID-19 vaccine should be given to people under the age of 30.[185] |
| 2021 | November 15 | Facility | BioNTech announces agreement with Senegal and Rwanda for the construction of the company’s first start-to-finish factories to make messenger RNA vaccines in Africa.[186][187][188][189] | ||
| 2021 | November | COVID-19 | BNT162b2 | Research | The full nucleotide sequences of the AstraZeneca and Pfizer/BioNTech vaccines are released by the United Kingdom Medicines and Healthcare Products Regulatory Agency, in response to a freedom of information request.[190][191] |
| 2021 | November 29 | COVID-19 | BNT162b2 | Product launch | Biontech announces the development of an adapted vaccine, aimed to serve as a precautionary measure while simultaneously carrying out research on the nature of the new SARS-CoV-2 Omicron variant.[192] |
| 2021 | December 20 | COVID-19 | BNT162b2 | Notable comment | Uğur Şahin says vaccines alone are not enough to fight SARS-CoV-2 Omicron variant, warning that other preventative measures need to be in place to stop the spread of the virus, especially with the emergence of the contagious variant.[193][194] |
| 2021 | December 22 | COVID-19 | BNT162b2 | Authorization (approval) | United Kingdom Medicines and Healthcare products Regulatory Agency approves use of Pfizer–BioNTech COVID-19 vaccine in 5 to 11-year olds, after finding that it is safe and effective.[195] |
| 2021 | November 29 | COVID-19 | BNT162b2 | Authorization (approval) | Taiwan's Ministry of Health and Welfare’s Advisory Committee for Immunization Practices (ACIP) approves second dose of the Pfizer–BioNTech COVID-19 vaccine for ages 12-17 and third dose for adults.[196] |
| 2021 | December 23 | COVID-19 | BNT162b2 | Recommendation | Hong Kong advisory panel recommends booster jab for those fully inoculated with the Pfizer–BioNTech COVID-19 vaccine. The government panel states that booster shots should been administered even if individuals are not among high-risk groups, as the SARS-CoV-2 Omicron variant by then accounts for about 80 per cent of imported infections.[197] |
| 2022 | January 5 | Shingles | Pipeline | BioNTech and Pfizer announce development of an mRNA-based vaccine for viral infection shingles, collaborating for the third time after the success of their COVID-19 vaccine based on the same technology. The vaccine would combine Pfizer's antigen technology and BioNTech's Messenger RNA platform technology.[198] | |
| 2022 | January 6 | COVID-19 | BNT162b2 | Recommendation | The US Centers for Disease Control and Prevention updates its recommendations for the Pfizer–BioNTech COVID-19 vaccine booster to include children as young as 12, at least five months after they finish the primary vaccine series.[199] |
| 2022 | January 7 | COVID-19 | BNT162b2 | Research | A study comparing Pfizer–BioNTech vaccine with Moderna's mRNA-1273 COVID-19 vaccine, suggests that concerning SARS-CoV-2 infection, hospitalization and death, the mRNA-1273 vaccine shows higher efficacy compared to the BNT162b2 vaccine.[200][201] |
| 2022 | January 10 | Partnership | BioNTech announces partnership with biopharmaceutical company Crescendo Biologics to develop multi-specific precision immunotherapies. BioNTech agrees to pay US$40 million upfront and promise up to $750 million in milestones for multiple programs. The initial term of the discovery collaboration is three years.[202][203][204] | ||
| 2022 | January 11 | COVID-19 | Partnership | BioNTech announces partnership with British AI-powered decision-making systemsprovider InstaDeep to develop a computational method that analyzes global sequencing data. Called the Early Warning System (EWS), it combines spike protein structural modeling with artificial intelligence algorithms to quickly flag potential high-risk COVID-19 variants.[205][206][207][208] | |
| 2022 | January 12 | COVID-19 | BNT162b2 | Recommendation | Hong Kong’s vaccine advisory panel recommends giving a partial dose of Pfizer–BioNTech COVID-19 vaccine to younger children, resorting to an “off-label use” amid supply shortages for a pediatric formulation. [209][210] |
| 2022 | January 18 | Cancer | BNT141 | Clinical trial | BioNTech starts a clinical trial with the purpose to evaluate mRNA encoded antibody therapeutic candidate BNT141, developed by the company for the treatment of solid tumors.[211] |
| 2022 | April | Cancer (non-small-cell lung carcinoma) | BNT116 | Clinical trial | BioNTech starts a first-in-human (FIH) trial for BNT116 aiming to establish the safety profile and a safe dose for BNT116 monotherapy as well as for BNT116 in combination with cemiplimab or in combination with docetaxel in patients with advanced or metastasized non-small cell lung cancer (NSCLC).[212] |
Numerical and visual data
Google Trends
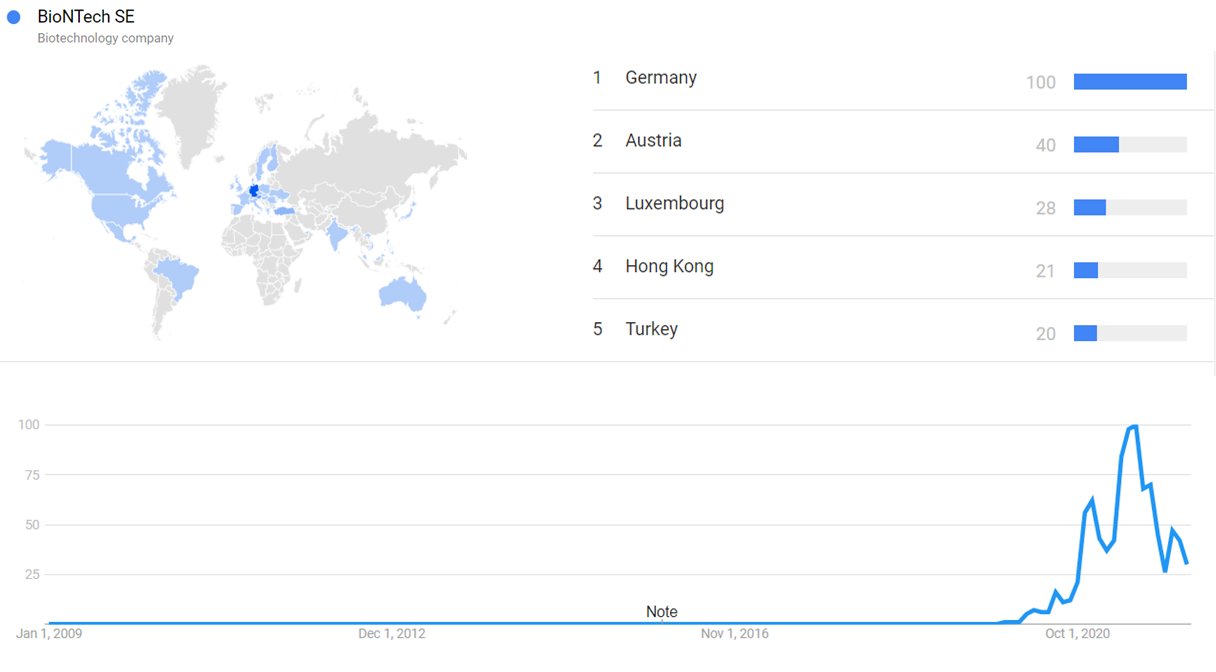
The chart below shows Google Trends data for BioNTech SE, from January 2009 to January 2022, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[213] The first notable increase on the chart (March 2020) is when BioNTech and Pfizer announce a COVID-19 vaccine partnership, and the second increase (October 2020) is after the success of human clinical trials. Since then, the fluctuation in interest has tracked the progress of the COVID-19 pandemic.
Google Finance
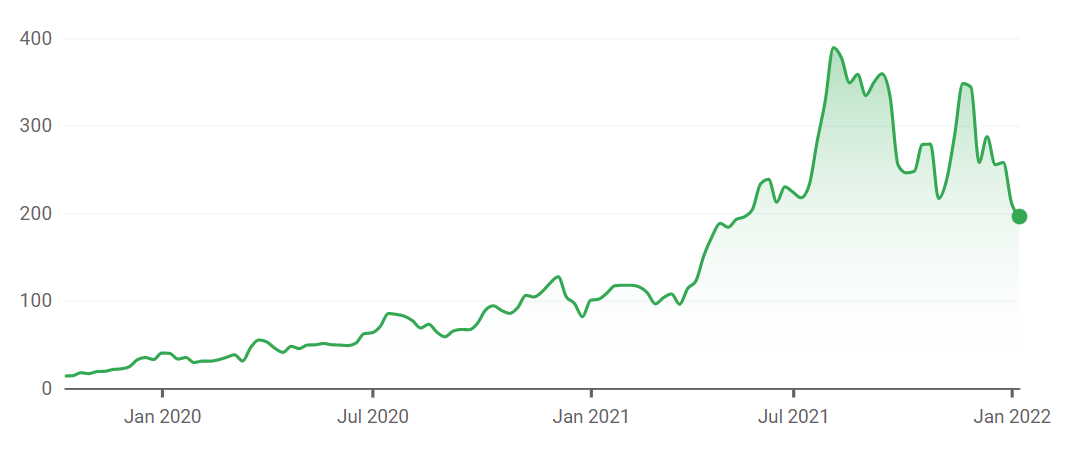
The image below shows BioNTech stock valuation in USD from IPO to January 14, 2022, when the screenshot was taken.[214]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by Sebastian.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
- List of COVID-19 vaccine authorizations
- [1] (clinical trials)
- [2] Ref name: [81]
- [[3]]
- Pfizer–BioNTech COVID-19 vaccine
- [4]
- [5]
Timeline update strategy
See also
External links
References
- ↑ "BioNTech: Be unique, treat individualized". BioNTech. Retrieved 26 February 2022.
- ↑ Fernández, Clara Rodríguez (15 November 2017). "US Biotech Unicorn Steps up Competition for BioNTech's mRNA Personalized Cancer Vaccine". Labiotech.eu. Retrieved 22 March 2022.
- ↑ Miller, Joe; Türeci, Özlem; Sahin, Ugur (1 February 2022). The Vaccine: Inside the Race to Conquer the COVID-19 Pandemic. St. Martin's Publishing Group. ISBN 978-1-250-28037-4.
- ↑ "NEWS Post - BioNTech IMFS". www.biontech-imfs.de. Retrieved 27 April 2022.
{{cite web}}: no-break space character in|title=at position 10 (help) - ↑ "TheraCode to Acquire JPT Peptide Technologies". GenomeWeb. 11 May 2009. Retrieved 27 April 2022.
- ↑ Miller, Joe; Türeci, Özlem; Sahin, Ugur (1 February 2022). The Vaccine: Inside the Race to Conquer the COVID-19 Pandemic. St. Martin's Publishing Group. ISBN 978-1-250-28037-4.
- ↑ 7.0 7.1 Lellouche, Julie (2 October 2014). "BioNTech and Ganymed Pharmaceuticals Open One of the Largest Research Facilities in Germany". Labiotech.eu. Retrieved 22 March 2022.
- ↑ "BioNTech: little-known German company behind the Covid-19 vaccine". German World. 7 October 2021. Retrieved 22 March 2022.
- ↑ "BioNTech secures $270 million in financing for further development of individualised immunotherapies". old.firstwordpharma.com. Retrieved 22 March 2022.
- ↑ "Heavy! Global mRNA Pharmaceutical Panorama". topic.echemi.com. Retrieved 21 April 2022.
- ↑ "BioNTech to Acquire Antibody Generation Unit of MAB Discovery" (PDF). biontech.de. Retrieved 22 March 2022.
- ↑ "BioNTech joins coronavirus vaccine race". cen.acs.org. Retrieved 27 April 2022.
- ↑ Rosenbaum, Leah. "BioNTech's Humble Billionaire CEO On The Next Era Of mRNA Vaccines". Forbes. Retrieved 27 April 2022.
- ↑ 14.0 14.1 Taylor, Phil (21 June 2021). "BioNTech takes its first cancer vaccine into phase 2 -". pharmaphorum.com. Retrieved 27 April 2022.
- ↑ 15.0 15.1 "Amid Covid vaccine success, BioNTech plans to return €2bn to shareholders". The Economic Times. Retrieved 27 April 2022.
- ↑ Kreiter, Sebastian; Selmi, Abderraouf; Diken, Mustafa; Sebastian, Martin; Osterloh, Phillip; Schild, Hansjörg; Huber, Christoph; Türeci, Özlem; Sahin, Ugur (1 January 2008). "Increased Antigen Presentation Efficiency by Coupling Antigens to MHC Class I Trafficking Signals". The Journal of Immunology. 180 (1): 309–318. doi:10.4049/jimmunol.180.1.309.
- ↑ 17.0 17.1 "BioNTech acquires Eufets". manufacturingchemist.com. Retrieved 25 April 2022.
- ↑ "BioNTech's founders: scientist couple in global spotlight". France 24. 13 November 2020. Retrieved 7 February 2022.
- ↑ "BioNTech's founders: scientist couple in global spotlight". France 24. 13 November 2020. Retrieved 25 April 2022.
- ↑ "SEC Filing | BioNTech". investors.biontech.de. Retrieved 25 April 2022.
- ↑ "BioNTech's founders: scientist couple in global spotlight". France 24. 13 November 2020. Retrieved 25 April 2022.
- ↑ Chapman, C. J.; Murray, A.; McElveen, J. E.; Sahin, U.; Luxemburger, U.; Türeci, Ö; Wiewrodt, R.; Barnes, A. C.; Robertson, J. F. (1 March 2008). "Autoantibodies in lung cancer: possibilities for early detection and subsequent cure". Thorax. 63 (3): 228–233. doi:10.1136/thx.2007.083592. ISSN 0040-6376.
- ↑ Koslowski, Michael; Sahin, Ugur; Dhaene, Karl; Huber, Christoph; Türeci, Özlem (1 May 2008). "MS4A12 Is a Colon-Selective Store-Operated Calcium Channel Promoting Malignant Cell Processes". Cancer Research. 68 (9): 3458–3466. doi:10.1158/0008-5472.CAN-07-5768.
- ↑ Sahin, Ugur; Koslowski, Michael; Dhaene, Karl; Usener, Dirk; Brandenburg, Gunda; Seitz, Gerhard; Huber, Christoph; Türeci, Özlem (1 December 2008). "Claudin-18 Splice Variant 2 Is a Pan-Cancer Target Suitable for Therapeutic Antibody Development". Clinical Cancer Research. 14 (23): 7624–7634. doi:10.1158/1078-0432.CCR-08-1547. ISSN 1078-0432.
- ↑ "BioNTech AG acquires EUFETS AG". www.bionity.com. Retrieved 25 April 2022.
- ↑ "The Biologics News and Reports Portal". pipelinereview. Retrieved 25 April 2022.
- ↑ Şahin, Ugur; BaŞer, Ulviye (2009). "Delay-Dependent Guaranteed Cost Control for T-S Fuzzy Descriptor Systems with Time Varying Delay". IFAC Proceedings Volumes. 42 (14): 402–407. doi:10.3182/20090901-3-RO-4009.00066.
- ↑ Sahin, Ugur; Türeci, Özlem; Pfreundschuh, Michael (17 August 2000). "Serologically identified tumour antigens as cancer vaccines". Cancer Vaccines and Immunotherapy: 195–206. doi:10.1017/CBO9780511527098.010.
- ↑ Kreiter, Sebastian; Selmi, Abderraouf; Diken, Mustafa; Koslowski, Michael; Britten, Cedrik M.; Huber, Christoph; Türeci, Ozlem; Sahin, Ugur (15 November 2010). "Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity". Cancer Research. 70 (22): 9031–9040. doi:10.1158/0008-5472.CAN-10-0699. ISSN 1538-7445.
- ↑ Klamp, Thorsten; Schumacher, Jens; Huber, Georg; Kühne, Christoph; Meissner, Ulrich; Selmi, Abderraouf; Hiller, Thomas; Kreiter, Sebastian; Markl, Jürgen; Türeci, Özlem; Sahin, Ugur (15 January 2011). "Highly specific auto-antibodies against claudin-18 isoform 2 induced by a chimeric HBcAg virus-like particle vaccine kill tumor cells and inhibit the growth of lung metastases". Cancer Research. 71 (2): 516–527. doi:10.1158/0008-5472.CAN-10-2292. ISSN 1538-7445.
- ↑ Castle, John C.; Kreiter, Sebastian; Diekmann, Jan; Löwer, Martin; van de Roemer, Niels; de Graaf, Jos; Selmi, Abderraouf; Diken, Mustafa; Boegel, Sebastian; Paret, Claudia; Koslowski, Michael; Kuhn, Andreas N.; Britten, Cedrik M.; Huber, Christoph; Türeci, Ozlem; Sahin, Ugur (1 March 2012). "Exploiting the mutanome for tumor vaccination". Cancer Research. 72 (5): 1081–1091. doi:10.1158/0008-5472.CAN-11-3722. ISSN 1538-7445.
- ↑ "Clinical First-in-human Dose Escalation Study Evaluating the Safety and Tolerability of Intranodal Administration of an RNA-based Cancer Vaccine Targeting Two Tumor-associated Antigens in Patients With Advanced Melanoma". clinicaltrials.gov. clinicaltrials.gov. 14 January 2020. Retrieved 21 February 2022.
- ↑ "First-in-human Study Evaluating the Safety, Tolerability and Immunogenicity of i.n. Administration of a Personalized Vaccination With IVAC MUTANOME Vaccine w/o Initial Treatment With RBL001/RBL002 Vaccine in Patients With Advanced Melanoma". clinicaltrials.gov. clinicaltrials.gov. 14 January 2020. Retrieved 21 February 2022.
- ↑ Castle, John C.; Loewer, Martin; Boegel, Sebastian; de Graaf, Jos; Bender, Christian; Tadmor, Arbel D.; Boisguerin, Valesca; Bukur, Thomas; Sorn, Patrick; Paret, Claudia; Diken, Mustafa; Kreiter, Sebastian; Türeci, Özlem; Sahin, Ugur (13 March 2014). "Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma". BMC genomics. 15: 190. doi:10.1186/1471-2164-15-190. ISSN 1471-2164.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ "CT26.CL25 | ATCC". www.atcc.org. Retrieved 24 February 2022.
- ↑ "Evaluation of the MammaTyper Kit, Ref 90020/90021 Performed on Clinical Material Obtained From Patients With Early Breast Cancer: Re-Examination of Tumor Material and Re-Evaluation of Patient Data From Patients Treated With Neo-adjuvant Therapy". clinicaltrials.gov. clinicaltrials.gov. 17 January 2017. Retrieved 21 February 2022.
- ↑ "Evaluation of the MammaTyperTM Kit, Ref 90020/90021 Performed on Clinical Material Obtained From Patients With Breast Cancer: Re-Examination of Tumor Material and Re-Evaluation of Patient Data of the FinHer-Study". clinicaltrials.gov. clinicaltrials.gov. 16 September 2016. Retrieved 21 February 2022.
- ↑ Kowalska, Joanna; Wypijewska del Nogal, Anna; Darzynkiewicz, Zbigniew M.; Buck, Janina; Nicola, Corina; Kuhn, Andreas N.; Lukaszewicz, Maciej; Zuberek, Joanna; Strenkowska, Malwina; Ziemniak, Marcin; Maciejczyk, Maciej; Bojarska, Elzbieta; Rhoads, Robert E.; Darzynkiewicz, Edward; Sahin, Ugur; Jemielity, Jacek (2014). "Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: versatile tools for manipulation of therapeutically relevant cap-dependent processes". Nucleic Acids Research. 42 (16): 10245–10264. doi:10.1093/nar/gku757. ISSN 1362-4962.
- ↑ Simon, Petra; Omokoko, Tana A.; Breitkreuz, Andrea; Hebich, Lisa; Kreiter, Sebastian; Attig, Sebastian; Konur, Abdo; Britten, Cedrik M.; Paret, Claudia; Dhaene, Karl; Türeci, Özlem; Sahin, Ugur (December 2014). "Functional TCR retrieval from single antigen-specific human T cells reveals multiple novel epitopes". Cancer Immunology Research. 2 (12): 1230–1244. doi:10.1158/2326-6066.CIR-14-0108. ISSN 2326-6074.
- ↑ Levin, Jennifer (2 October 2014). "BioNTech AG and Ganymed Pharmaceuticals AG Open New Research Headquarters in Mainz, Germany". Fierce Biotech. Retrieved 22 March 2022.
- ↑ "A Phase I Trial of Actively Personalized Peptide Vaccinations Plus Immunomodulators in Patients With Newly Diagnosed Glioblastoma Concurrent to First Line Temozolomide Maintenance Therapy". clinicaltrials.gov. clinicaltrials.gov. 6 August 2018. Retrieved 22 February 2022.
- ↑ Omokoko, Tana; Simon, Petra; Türeci, Özlem; Sahin, Ugur (19 March 2015). "Retrieval of functional TCRs from single antigen-specific T cells: Toward individualized TCR-engineered therapies". OncoImmunology. 4 (7): e1005523. doi:10.1080/2162402X.2015.1005523. ISSN 2162-402X.
- ↑ "Clinical First-in-human Dose Escalation Study Evaluating the Safety and Tolerability of Intravenous Administration of a Tetravalent RNA-lipoplex Cancer Vaccine Targeting the Tumor-associated Antigens NY-ESO-1, Tyrosinase, MAGE-A3, and TPTE in Patients With Advanced Melanoma". clinicaltrials.gov. clinicaltrials.gov. 23 December 2021. Retrieved 21 February 2022.
- ↑ Poleganov, Marco Alexander; Eminli, Sarah; Beissert, Tim; Herz, Stephanie; Moon, Jung-Il; Goldmann, Johanna; Beyer, Arianne; Heck, Rosario; Burkhart, Isabell; Barea Roldan, Diana; Türeci, Özlem; Yi, Kevin; Hamilton, Brad; Sahin, Ugur (November 2015). "Efficient Reprogramming of Human Fibroblasts and Blood-Derived Endothelial Progenitor Cells Using Nonmodified RNA for Reprogramming and Immune Evasion". Human Gene Therapy. 26 (11): 751–766. doi:10.1089/hum.2015.045. ISSN 1557-7422.
- ↑ "Phase 1 Safety and Tolerability Study of Human Monoclonal Antibody 5B1 (MVT-5873) With Expansion in Subjects With Pancreatic Cancer or Other CA19-9 Positive Malignancies". clinicaltrials.gov. clinicaltrials.gov. 4 February 2022. Retrieved 21 February 2022.
- ↑ Kreiter, Sebastian; Vormehr, Mathias; van de Roemer, Niels; Diken, Mustafa; Löwer, Martin; Diekmann, Jan; Boegel, Sebastian; Schrörs, Barbara; Vascotto, Fulvia; Castle, John C.; Tadmor, Arbel D.; Schoenberger, Stephen P.; Huber, Christoph; Türeci, Özlem; Sahin, Ugur (30 April 2015). "Mutant MHC class II epitopes drive therapeutic immune responses to cancer". Nature. 520 (7549): 692–696. doi:10.1038/nature14426. ISSN 1476-4687.
- ↑ AG, BioNTech. "Bayer and BioNTech Join Forces to Develop Innovative New mRNA Vaccines and Therapeutics for Animal Health Applications". www.prnewswire.com. Retrieved 7 February 2022.
- ↑ Kranz, Lena M.; Diken, Mustafa; Haas, Heinrich; Kreiter, Sebastian; Loquai, Carmen; Reuter, Kerstin C.; Meng, Martin; Fritz, Daniel; Vascotto, Fulvia; Hefesha, Hossam; Grunwitz, Christian; Vormehr, Mathias; Hüsemann, Yves; Selmi, Abderraouf; Kuhn, Andreas N.; Buck, Janina; Derhovanessian, Evelyna; Rae, Richard; Attig, Sebastian; Diekmann, Jan; Jabulowsky, Robert A.; Heesch, Sandra; Hassel, Jessica; Langguth, Peter; Grabbe, Stephan; Huber, Christoph; Türeci, Özlem; Sahin, Ugur (16 June 2016). "Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy". Nature. 534 (7607): 396–401. doi:10.1038/nature18300. ISSN 1476-4687. Retrieved 8 February 2022.
- ↑ "Phase 1 Study of 89Zr-DFO-HuMab-5B1 (MVT-2163) With HuMab-5B1 (MVT-5873) in Patients With Pancreatic Cancer or Other CA19-9 Positive Malignancies". clinicaltrials.gov. clinicaltrials.gov. 26 August 2021. Retrieved 21 February 2022.
- ↑ Writer, GEN Staff (21 September 2016). "Genentech, BioNTech to Partner on mRNA Cancer Vaccines". GEN - Genetic Engineering and Biotechnology News. Retrieved 4 February 2022.
- ↑ AG, BioNTech. "BioNTech to Enter Into Worldwide Strategic Collaboration With Genentech to Develop Individualized mRNA Cancer Therapies". www.prnewswire.com. Retrieved 7 February 2022.
- ↑ Grabbe, Stephan; Haas, Heinrich; Diken, Mustafa; Kranz, Lena M.; Langguth, Peter; Sahin, Ugur (October 2016). "Translating nanoparticulate-personalized cancer vaccines into clinical applications: case study with RNA-lipoplexes for the treatment of melanoma". Nanomedicine (London, England). 11 (20): 2723–2734. doi:10.2217/nnm-2016-0275. ISSN 1748-6963.
- ↑ "First-in-human Clinical Study With RNA-Immunotherapy Combination of IVAC_W_bre1_uID and IVAC_M_uID for Individualized Tumour Therapy in Triple Negative Breast Cancer Patients". clinicaltrials.gov. clinicaltrials.gov. 8 February 2021. Retrieved 21 February 2022.
- ↑ "Antigen-targeted Personalized Breast Cancer Vaccine | Semantic Scholar". www.semanticscholar.org. Retrieved 3 March 2022.
- ↑ "T-cell responses induced by an individualized neoantigen specific immune therapy in post (neo)adjuvant patients with triple negative breast cancer | OncologyPRO". oncologypro.esmo.org. Retrieved 3 March 2022.
- ↑ "An Open-label, Phase IB Study of NEO-PV-01 + Adjuvant With Nivolumab in Patients With Melanoma, Non-Small Cell Lung Carcinoma or Transitional Cell Carcinoma of the Bladder". clinicaltrials.gov. clinicaltrials.gov. 24 February 2021. Retrieved 21 February 2022.
- ↑ "NEO PV 01 - AdisInsight". adisinsight.springer.com. Retrieved 22 March 2022.
- ↑ "Therapeutic HPV Vaccine Trial +/- Anti-CD40 in HPV-driven Squamous Cell Carcinoma". clinicaltrials.gov. clinicaltrials.gov. 5 May 2021. Retrieved 22 February 2022.
- ↑ Pardi, Norbert; Hogan, Michael J.; Pelc, Rebecca S.; Muramatsu, Hiromi; Andersen, Hanne; DeMaso, Christina R.; Dowd, Kimberly A.; Sutherland, Laura L.; Scearce, Richard M.; Parks, Robert; Wagner, Wendeline; Granados, Alex; Greenhouse, Jack; Walker, Michelle; Willis, Elinor; Yu, Jae-Sung; McGee, Charles E.; Sempowski, Gregory D.; Mui, Barbara L.; Tam, Ying K.; Huang, Yan-Jang; Vanlandingham, Dana; Holmes, Veronica M.; Balachandran, Harikrishnan; Sahu, Sujata; Lifton, Michelle; Higgs, Stephen; Hensley, Scott E.; Madden, Thomas D.; Hope, Michael J.; Karikó, Katalin; Santra, Sampa; Graham, Barney S.; Lewis, Mark G.; Pierson, Theodore C.; Haynes, Barton F.; Weissman, Drew (9 March 2017). "Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination". Nature. 543 (7644): 248–251. doi:10.1038/nature21428. ISSN 1476-4687.
- ↑ "Phase I, Open-Label, Multi-Center, Dose Escalation With Expansion Trial of 177Lu Human Monoclonal Antibody 5B1 (MVT-1075) in Combination With a Blocking Dose of MVT-5873 as Radioimmunotherapy in Relapse/Refractory Subjects With Pancreatic Cancer or Other CA19-9 Positive Malignancies". clinicaltrials.gov. clinicaltrials.gov. 15 April 2021. Retrieved 21 February 2022.
- ↑ "A Phase I Study of MVT-5873 Alone or with Chemotherapy in Patients with Pancreatic Cancer and Other CA19-9 Positive Tumors | Memorial Sloan Kettering Cancer Center". www.mskcc.org. Retrieved 15 April 2022.
- ↑ Sahin, Ugur; Derhovanessian, Evelyna; Miller, Matthias; Kloke, Björn-Philipp; Simon, Petra; Löwer, Martin; Bukur, Valesca; Tadmor, Arbel D.; Luxemburger, Ulrich; Schrörs, Barbara; Omokoko, Tana; Vormehr, Mathias; Albrecht, Christian; Paruzynski, Anna; Kuhn, Andreas N.; Buck, Janina; Heesch, Sandra; Schreeb, Katharina H.; Müller, Felicitas; Ortseifer, Inga; Vogler, Isabel; Godehardt, Eva; Attig, Sebastian; Rae, Richard; Breitkreuz, Andrea; Tolliver, Claudia; Suchan, Martin; Martic, Goran; Hohberger, Alexander; Sorn, Patrick; Diekmann, Jan; Ciesla, Janko; Waksmann, Olga; Brück, Alexandra-Kemmer; Witt, Meike; Zillgen, Martina; Rothermel, Andree; Kasemann, Barbara; Langer, David; Bolte, Stefanie; Diken, Mustafa; Kreiter, Sebastian; Nemecek, Romina; Gebhardt, Christoffer; Grabbe, Stephan; Höller, Christoph; Utikal, Jochen; Huber, Christoph; Loquai, Carmen; Türeci, Özlem (13 July 2017). "Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer". Nature. 547 (7662): 222–226. doi:10.1038/nature23003. ISSN 1476-4687.
- ↑ Stadler, Christiane R.; Bähr-Mahmud, Hayat; Celik, Leyla; Hebich, Bernhard; Roth, Alexandra S.; Roth, René P.; Karikó, Katalin; Türeci, Özlem; Sahin, Ugur (July 2017). "Elimination of large tumors in mice by mRNA-encoded bispecific antibodies". Nature Medicine. 23 (7): 815–817. doi:10.1038/nm.4356. ISSN 1546-170X.
- ↑ SE, BioNTech (6 September 2017). "BioNTech AG Enters into Licensing Agreement with CELLSCRIPT, LLC as it Advances Development of Messenger RNA Encoding Bispecific Antibodies and other Therapeutic Proteins". GlobeNewswire News Room. Retrieved 4 February 2022.
- ↑ "A Study of Autogene Cevumeran (RO7198457) as a Single Agent and in Combination With Atezolizumab in Participants With Locally Advanced or Metastatic Tumors". clinicaltrials.gov. Retrieved 22 February 2022.
- ↑ SE, BioNTech (4 January 2018). "BioNTech Secures USD 270 Million in Series A Financing". GlobeNewswire News Room. Retrieved 4 February 2022.
- ↑ Schumacher, Jens; Bacic, Tijana; Staritzbichler, René; Daneschdar, Matin; Klamp, Thorsten; Arnold, Philipp; Jägle, Sabrina; Türeci, Özlem; Markl, Jürgen; Sahin, Ugur (13 April 2018). "Enhanced stability of a chimeric hepatitis B core antigen virus-like-particle (HBcAg-VLP) by a C-terminal linker-hexahistidine-peptide". Journal of Nanobiotechnology. 16 (1): 39. doi:10.1186/s12951-018-0363-0. ISSN 1477-3155.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ S.L, Rekom Biotech. "HBcAg antigen - Rekom Biotech". www.rekombiotech.com. Retrieved 22 March 2022.
- ↑ "An Open-Label, Phase 1B Study of NEO-PV-01 With Pembrolizumab Plus Chemotherapy in Patients With Advanced or Metastatic Nonsquamous Non-small Cell Lung Cancer". clinicaltrials.gov. clinicaltrials.gov. 24 February 2021. Retrieved 21 February 2022.
- ↑ Ott, P.A.; Govindan, R.; Naing, A.; Friedlander, T.W.; Margolin, K.; Lin, J.J.; Bhardwaj, N.; Hellman, M.D.; Srinivasan, L.; Greshock, J.; Moles, M.; Gaynor, R.B.; Goldstein, M.J.; Hu-Lieskovan, S. (October 2018). "A personal neoantigen vaccine, NEO-PV-01, with anti-PD1 induces broad de novo anti-tumor immunity in patients with metastatic melanoma, NSCLC, and bladder cancer". Annals of Oncology. 29: viii400. doi:10.1093/annonc/mdy288.
- ↑ SE, BioNTech (29 May 2018). "BioNTech Appoints Dr. Özlem Türeci as Chief Medical Officer". GlobeNewswire News Room. Retrieved 4 February 2022.
- ↑ "BioNTech and Genevant Sciences Sign Strategic mRNA-Focused Partnership in Rare Diseases". www.businesswire.com. 10 July 2018. Retrieved 4 February 2022.
- ↑ "Pfizer pays BioNTech $120M upfront to form mRNA flu vaccine pact". FierceBiotech. Retrieved 4 February 2022.
- ↑ "BioNTech signs $425m flu vaccine deal with Pfizer". Pharmaceutical Technology. 16 August 2018. Retrieved 4 February 2022.
- ↑ Dale, Alex (9 January 2018). "Scancell and BioNTech Will Combine Their Expertise to Fight Cancer". Labiotech.eu. Retrieved 4 February 2022.
- ↑ "An Open-label, Phase 1B Study of NEO-PV-01 + CD40 Agonist Antibody (APX005M) or Ipilimumab With Nivolumab in Patients With Advanced or Metastatic Melanoma". clinicaltrials.gov. clinicaltrials.gov. 1 September 2020. Retrieved 21 February 2022.
- ↑ "BioNTech taps Drew Weissmann's UPenn lab to expand into infectious disease mRNA vaccines". FierceBiotech. Retrieved 4 February 2022.
- ↑ "BioNTech: MammaTyper® Validated as Predictor for Response After Neoadjuvant Chemotherapy (NACT) in Patients with Early Breast Cancer (BC)". www.b3cnewswire.com. Retrieved 4 February 2022.
- ↑ "A Phase 1 First-in-Human Dose Escalation and Expansion Study for the Evaluation of Safety, Pharmacokinetics, Pharmacodynamics and Anti-tumor Activity of SAR441000 Administered Intratumorally as Monotherapy and in Combination With Cemiplimab in Patients With Advanced Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 13 January 2022. Retrieved 22 February 2022.
- ↑ Bechter, Oliver; Utikal, Jochen; Baurain, Jean-Francois; Massard, Christophe; Sahin, Ugur; Derhovanessian, Evelyna; Ozoux, Marie-Laure; Marpadga, Rahul; Imedio, Esteban-Rodrigo; Acquavella, Nicolas; Loquai, Carmen (November 2020). "391 A first-in-human study of intratumoral SAR441000, an mRNA mixture encoding IL-12sc, interferon alpha2b, GM-CSF and IL-15sushi as monotherapy and in combination with cemiplimab in advanced solid tumors". Regular and young investigator award abstracts: A237.2–A238. doi:10.1136/jitc-2020-SITC2020.0391.
- ↑ 81.0 81.1 81.2 "BioNTech" (PDF). biontech.de. Retrieved 24 February 2022.
- ↑ "Sanofi invests €80M in BioNTech as cancer mRNA hits clinic". FierceBiotech. Retrieved 4 February 2022.
- ↑ Smith, Jonathan (7 January 2019). "Sanofi Invests €80M in mRNA Cancer Vaccine Company". Labiotech.eu. Retrieved 4 February 2022.
- ↑ "BioNTech expands cancer research collaboration with Sanofi". www.pharmaceutical-business-review.com. Retrieved 7 February 2022.
- ↑ "BioNTech Announces Corporate Form Transition from AG to SE; Welcomes Ryan Richardson as Senior Vice President Corporate Development and Strategy and Appoints Dr. Ulrich Wandschneider as Member of Supervisory Board". www.businesswire.com. 4 January 2019. Retrieved 4 February 2022.
- ↑ "A Phase II, Open-Label, Multicenter, Randomized Study Of The Efficacy And Safety Of RO7198457 In Combination With Pembrolizumab Versus Pembrolizumab In Patients With Previously Untreated Advanced Melanoma". clinicaltrials.gov. clinicaltrials.gov. 2 February 2022. Retrieved 22 February 2022.
- ↑ Braiteh, Fadi; LoRusso, Patricia; Balmanoukian, Ani; Klempner, Sam; Camidge, D R.; Hellmann, Matthew; Gordon, Michael; Bendell, Johanna; Mueller, Lars; Sabado, Rachel; Twomey, Patrick; Delamarre, Leila; Huang, Jack; Yadav, Mahesh; Zhang, Jingbin; McDonald, Patrick; Müller, Felicitas; Derhovanessian, Evelyna; Türeci, Özlem; Sahin, Ugur; Siu, Lillian (15 August 2020). "Abstract CT169: A phase Ia study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in patients with locally advanced or metastatic solid tumors". Tumor Biology: CT169 – CT169. doi:10.1158/1538-7445.AM2020-CT169.
- ↑ "BioNTech to Acquire Antibody Generation Unit of MAB Discovery". www.businesswire.com. 23 January 2019. Retrieved 4 February 2022.
- ↑ "Biontech burrows into antibody diversity with Mab Discovery unit buy". bioworld.com. Retrieved 7 February 2022.
- ↑ "BioNTech Buys MAB Discovery's Antibody Generation Unit". BioSpace. Retrieved 7 February 2022.
- ↑ Vascotto, Fulvia; Petschenka, Jutta; Walzer, Kerstin C.; Vormehr, Mathias; Brkic, Magdalena; Strobl, Stefan; Rösemann, Roman; Diken, Mustafa; Kreiter, Sebastian; Türeci, Özlem; Sahin, Ugur (2019). "Intravenous delivery of the toll-like receptor 7 agonist SC1 confers tumor control by inducing a CD8+ T cell response". Oncoimmunology. 8 (7): 1601480. doi:10.1080/2162402X.2019.1601480. ISSN 2162-4011.
- ↑ Chi, Huju; Li, Chunman; Zhao, Flora Sha; Zhang, Li; Ng, Tzi Bun; Jin, Guangyi; Sha, Ou (31 May 2017). "Anti-tumor Activity of Toll-Like Receptor 7 Agonists". Frontiers in Pharmacology. 8: 304. doi:10.3389/fphar.2017.00304.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ "BioNTech snaps up struggling MabVax's lead asset, labs, eyeing San Diego R&D site". FierceBiotech.
- ↑ AG, BIOCOM. "BioNtech aquires antibody assets from MabVax Therapeutics". european-biotechnology.com. Retrieved 7 February 2022.
- ↑ "First-in-human, Open-label, Dose-escalation Trial With Expansion Cohorts to Evaluate Safety of GEN1046 in Subjects With Malignant Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 14 January 2022. Retrieved 22 February 2022.
- ↑ "Next-Generation Bispecific Antibody Shows Early Clinical Activity in Advanced Solid Tumors". OncLive. Retrieved 20 March 2022.
- ↑ "Next Generation Technology Platforms | Genmab". www.genmab.com. Retrieved 20 March 2022.
- ↑ "BioNTech and Genmab Initiate First-In-Human Phase I/IIa Trial of Bispecific Antibody DuoBody®-PD-L1x4-1BB in Solid Tumors". BioSpace. Retrieved 4 February 2022.
- ↑ "BioNTech to Acquire Antibody Generation Unit of MAB Discovery | BioNTech". investors.biontech.de. Retrieved 26 February 2022.
- ↑ Smith, Jonathan (9 July 2019). "BioNTech Raises Whopping €290M to Fund Personalized mRNA Vaccines for Cancer". Labiotech.eu. Retrieved 4 February 2022.
- ↑ "BioNTech raises €290m for mRNA cancer vaccine platform". PMLive. 10 July 2019. Retrieved 7 February 2022.
- ↑ "BioNTech Announces New Collaboration to Develop HIV and Tuberculosis Programs". www.businesswire.com. 4 September 2019. Retrieved 4 February 2022.
- ↑ "BioNTech partners with Bill and Melinda Gates Foundation, scoring $55M equity investment; BeiGene brushes off short attack". Endpoints News. Retrieved 7 February 2022.
- ↑ "A First-in-Human, Open-label, Dose-escalation Trial With Expansion Cohorts to Evaluate Safety and Anti-tumor Activity of GEN1042 in Subjects With Malignant Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 28 September 2021. Retrieved 22 February 2022.
- ↑ "GEN 1042 - AdisInsight". adisinsight.springer.com. Retrieved 20 April 2022.
- ↑ PHD, Hans W. Nijman, MD (11 May 2021). "Ovarian Cancer Treatment With a Liposome Formulated mRNA Vaccine in Combination With (Neo-)Adjuvant Chemotherapy". clinicaltrials.gov. clinicaltrials.gov. Retrieved 22 February 2022.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ "BioNTech SE Provides Update on Corporate Progress and Third Quarter 2019 Financial Results". www.sec.gov. Retrieved 16 April 2022.
- ↑ "Press corner". European Commission - European Commission. Retrieved 14 January 2022.
- ↑ "First-in-human, Dose Titration and Expansion Trial to Evaluate Safety, Immunogenicity and Preliminary Efficacy of W_pro1 (BNT112) Monotherapy and in Combination With Cemiplimab in Patients With Prostate Cancer". clinicaltrials.gov. clinicaltrials.gov. 16 February 2022. Retrieved 21 February 2022.
- ↑ Reinhard, Katharina; Rengstl, Benjamin; Oehm, Petra; Michel, Kristina; Billmeier, Arne; Hayduk, Nina; Klein, Oliver; Kuna, Kathrin; Ouchan, Yasmina; Wöll, Stefan; Christ, Elmar; Weber, David; Suchan, Martin; Bukur, Thomas; Birtel, Matthias; Jahndel, Veronika; Mroz, Karolina; Hobohm, Kathleen; Kranz, Lena; Diken, Mustafa; Kühlcke, Klaus; Türeci, Özlem; Sahin, Ugur (24 January 2020). "An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors". Science. doi:10.1126/science.aay5967.
- ↑ "Pfizer and BioNTech to Co-develop Potential COVID-19 Vaccine". BioNTech. March 17, 2020. Retrieved May 1, 2022.
- ↑ "Pfizer and BioNTech to Co-develop Potential COVID-19 Vaccine". Pfizer. March 17, 2020. Retrieved May 1, 2022.
- ↑ Mulligan, Mark J.; Lyke, Kirsten E.; Kitchin, Nicholas; Absalon, Judith; Gurtman, Alejandra; Lockhart, Stephen; Neuzil, Kathleen; Raabe, Vanessa; Bailey, Ruth; Swanson, Kena A.; Li, Ping; Koury, Kenneth; Kalina, Warren; Cooper, David; Fontes-Garfias, Camila; Shi, Pei-Yong; Türeci, Özlem; Tompkins, Kristin R.; Walsh, Edward E.; Frenck, Robert; Falsey, Ann R.; Dormitzer, Philip R.; Gruber, William C.; Şahin, Uğur; Jansen, Kathrin U. (October 2020). "Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults". Nature. 586 (7830): 589–593. doi:10.1038/s41586-020-2639-4. ISSN 1476-4687.
- ↑ "A Multi-site, Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of Four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-2019 Using Different Dosing Regimens in Healthy and Immunocompromised Adults". clinicaltrials.gov. clinicaltrials.gov. 12 January 2022. Retrieved 21 February 2022.
- ↑ "A PHASE 1/2/3, PLACEBO-CONTROLLED, RANDOMIZED, OBSERVER-BLIND, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, IMMUNOGENICITY, AND EFFICACY OF SARS-COV-2 RNA VACCINE CANDIDATES AGAINST COVID-19 IN HEALTHY INDIVIDUALS". clinicaltrials.gov. clinicaltrials.gov. 1 February 2022. Retrieved 21 February 2022.
- ↑ "Phase 1/2a, First-in-human, Open-label, Dose-escalation Trial With Expansion Cohorts to Evaluate Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy of BNT411 as a Monotherapy in Patients With Solid Tumors and in Combination With Atezolizumab, Carboplatin and Etoposide in Patients With Chemotherapy-naïve Extensive-stage Small Cell Lung Cancer (ES-SCLC)". clinicaltrials.gov. clinicaltrials.gov. 22 July 2021. Retrieved 21 February 2022.
- ↑ "BNT 411 - AdisInsight". adisinsight.springer.com. Retrieved 3 March 2022.
- ↑ "Britain secures 90 million possible COVID-19 vaccine doses from Pfizer/BioNTech, Valneva". reuters.com. Retrieved 13 January 2022.
- ↑ SE, BioNTech (27 July 2020). "BioNTech Announces Closing of $512 Million Underwritten Offering of American Depositary Shares". GlobeNewswire News Room. Retrieved 4 February 2022.
- ↑ "Safety and Immunogenicity of SARS-CoV-2 mRNA Vaccine (BNT162b1) in Chinese Healthy Subjects: A Phase I, Randomized, Placebo-controlled, Observer-blind Study". clinicaltrials.gov. clinicaltrials.gov. 5 November 2021. Retrieved 21 February 2022.
- ↑ Sahin, Ugur; Oehm, Petra; Derhovanessian, Evelyna; Jabulowsky, Robert A.; Vormehr, Mathias; Gold, Maike; Maurus, Daniel; Schwarck-Kokarakis, Doreen; Kuhn, Andreas N.; Omokoko, Tana; Kranz, Lena M.; Diken, Mustafa; Kreiter, Sebastian; Haas, Heinrich; Attig, Sebastian; Rae, Richard; Cuk, Katarina; Kemmer-Brück, Alexandra; Breitkreuz, Andrea; Tolliver, Claudia; Caspar, Janina; Quinkhardt, Juliane; Hebich, Lisa; Stein, Malte; Hohberger, Alexander; Vogler, Isabel; Liebig, Inga; Renken, Stephanie; Sikorski, Julian; Leierer, Melanie; Müller, Verena; Mitzel-Rink, Heidrun; Miederer, Matthias; Huber, Christoph; Grabbe, Stephan; Utikal, Jochen; Pinter, Andreas; Kaufmann, Roland; Hassel, Jessica C.; Loquai, Carmen; Türeci, Özlem (September 2020). "An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma". Nature. 585 (7823): 107–112. doi:10.1038/s41586-020-2537-9. ISSN 1476-4687.
- ↑ "Pfizer, BioNTech to supply 120 million doses of coronavirus vaccine to Japan". Reuters. 31 July 2020. Retrieved 13 January 2022.
- ↑ SE, BioNTech (5 August 2020). "Pfizer and BioNTech to Supply Canada with their BNT162 mRNA-Based Vaccine Candidate". GlobeNewswire News Room. Retrieved 13 January 2022.
- ↑ "A Multi-site, Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of a Prophylactic SARS-CoV-2 RNA Vaccine (BNT162b3) Against COVID-19 Using Different Dosing Regimens in Healthy Adults". clinicaltrials.gov. clinicaltrials.gov. 12 January 2022. Retrieved 21 February 2022.
- ↑ "Phase I/IIa, First-in-human (FIH), Open-label, Dose Escalation Trial With Expansion Cohorts to Evaluate Safety and Preliminary Efficacy of CLDN6 CAR-T +/- CLDN6 RNA-LPX in Patients With CLDN6-positive Relapsed or Refractory Advanced Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 9 June 2021. Retrieved 21 February 2022.
- ↑ "CLDN6-Targeting CAR T-Cell Therapy Produces Acceptable Responses in Solid Tumors". Cancer Network. Retrieved 20 April 2022.
- ↑ Fortuna, Gerardo (8 January 2021). "Commission takes evasive action over Germany's vaccine side deal". www.euractiv.com. Retrieved 13 January 2022.
- ↑ Sahin, Ugur; Muik, Alexander; Derhovanessian, Evelyna; Vogler, Isabel; Kranz, Lena M.; Vormehr, Mathias; Baum, Alina; Pascal, Kristen; Quandt, Jasmin; Maurus, Daniel; Brachtendorf, Sebastian; Lörks, Verena; Sikorski, Julian; Hilker, Rolf; Becker, Dirk; Eller, Ann-Kathrin; Grützner, Jan; Boesler, Carsten; Rosenbaum, Corinna; Kühnle, Marie-Cristine; Luxemburger, Ulrich; Kemmer-Brück, Alexandra; Langer, David; Bexon, Martin; Bolte, Stefanie; Karikó, Katalin; Palanche, Tania; Fischer, Boris; Schultz, Armin; Shi, Pei-Yong; Fontes-Garfias, Camila; Perez, John L.; Swanson, Kena A.; Loschko, Jakob; Scully, Ingrid L.; Cutler, Mark; Kalina, Warren; Kyratsous, Christos A.; Cooper, David; Dormitzer, Philip R.; Jansen, Kathrin U.; Türeci, Özlem (October 2020). "COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses". Nature. 586 (7830): 594–599. doi:10.1038/s41586-020-2814-7. ISSN 1476-4687.
- ↑ Walsh, Edward E.; Frenck, Robert W.; Falsey, Ann R.; Kitchin, Nicholas; Absalon, Judith; Gurtman, Alejandra; Lockhart, Stephen; Neuzil, Kathleen; Mulligan, Mark J.; Bailey, Ruth; Swanson, Kena A.; Li, Ping; Koury, Kenneth; Kalina, Warren; Cooper, David; Fontes-Garfias, Camila; Shi, Pei-Yong; Türeci, Özlem; Tompkins, Kristin R.; Lyke, Kirsten E.; Raabe, Vanessa; Dormitzer, Philip R.; Jansen, Kathrin U.; Şahin, Uğur; Gruber, William C. (17 December 2020). "Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates". New England Journal of Medicine. 383 (25): 2439–2450. doi:10.1056/NEJMoa2027906. ISSN 0028-4793.
- ↑ "A PHASE 1/2, PLACEBO-CONTROLLED, RANDOMIZED, AND OBSERVER-BLIND STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND IMMUNOGENICITY OF A SARS-COV-2 RNA VACCINE CANDIDATE AGAINST COVID-19 IN HEALTHY JAPANESE ADULTS". clinicaltrials.gov. clinicaltrials.gov. 17 December 2021. Retrieved 21 February 2022.
- ↑ "Update on our COVID-19 vaccine development program with BNT162b2". investors.biontech.de. Retrieved 14 January 2022.
- ↑ "Australia Secures a further 50 Million Doses of COVID-19 Vaccine | Prime Minister of Australia". www.pm.gov.au. Retrieved 13 January 2022.
- ↑ "Coronavirus: EU buys 300m doses of BioNTech-Pfizer vaccine". BBC News. 11 November 2020. Retrieved 13 January 2022.
- ↑ "Pfizer and BioNTech to Submit Emergency Use Authorization Request Today to the U.S. FDA for COVID-19 Vaccine". pfizer.com. Retrieved 18 February 2022.
- ↑ "An Open-label, Phase I Study of NEO-PTC-01 in Patients With Advanced or Metastatic Melanoma". clinicaltrials.gov. clinicaltrials.gov. 2 December 2021. Retrieved 21 February 2022.
- ↑ Inc, Neon Therapeutics (30 April 2020). "Neon Therapeutics Announces Acceptance of European Clinical Trial Authorization Application for NEO-PTC-01". GlobeNewswire News Room. Retrieved 15 April 2022.
{{cite web}}:|last1=has generic name (help) - ↑ "Information for Healthcare Professionals on COVID-19 Vaccine Pfizer/BioNTech (Regulation 174)". GOV.UK. Retrieved 14 January 2022.
- ↑ "Conditions of Authorisation for COVID-19 Vaccine Pfizer/BioNTech (Regulation 174)". GOV.UK. Retrieved 14 January 2022.
- ↑ "UK medicines regulator gives approval for first UK COVID-19 vaccine". GOV.UK. Retrieved 14 January 2022.
- ↑ "UK approves Pfizer/BioNTech Covid vaccine for rollout next week". the Guardian. 2 December 2020. Retrieved 14 January 2022.
- ↑ "Swissmedic grants authorisation for the first COVID-19 vaccine in Switzerland". www.bag.admin.ch. Retrieved 14 January 2022.
- ↑ "Safety and Immunogenicity of SARS-CoV-2 mRNA Vaccine (BNT162b2) in Chinese Healthy Population: A Phase II, Randomized, Placebo-controlled, Observer-blinded Study". clinicaltrials.gov. clinicaltrials.gov. 12 February 2021. Retrieved 21 February 2022.
- ↑ Polack, Fernando P.; Thomas, Stephen J.; Kitchin, Nicholas; Absalon, Judith; Gurtman, Alejandra; Lockhart, Stephen; Perez, John L.; Pérez Marc, Gonzalo; Moreira, Edson D.; Zerbini, Cristiano; Bailey, Ruth; Swanson, Kena A.; Roychoudhury, Satrajit; Koury, Kenneth; Li, Ping; Kalina, Warren V.; Cooper, David; Frenck, Robert W.; Hammitt, Laura L.; Türeci, Özlem; Nell, Haylene; Schaefer, Axel; Ünal, Serhat; Tresnan, Dina B.; Mather, Susan; Dormitzer, Philip R.; Şahin, Uğur; Jansen, Kathrin U.; Gruber, William C. (31 December 2020). "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine". New England Journal of Medicine. 383 (27): 2603–2615. doi:10.1056/NEJMoa2034577. ISSN 0028-4793.
- ↑ Thomas, Katie; LaFraniere, Sharon; Weiland, Noah; Goodnough, Abby; Haberman, Maggie (12 December 2020). "F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away". The New York Times. Retrieved 14 January 2022.
- ↑ "EMA recommends first COVID-19 vaccine for authorisation in the EU". ema.europa.eu. Retrieved 13 January 2022.
- ↑ Costa, José María (23 December 2020). "Coronavirus en la Argentina: la Anmat aprobó el uso de emergencia de la vacuna de Pfizer". La Nación (in español). Retrieved 14 January 2022.
- ↑ "'Window of hope': Europe begins to launch COVID-19 vaccinations". Reuters. 27 December 2020. Retrieved 13 January 2022.
- ↑ Moghimi SM (March 2021). "Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines". Molecular Therapy. 29 (3): 898–900. doi:10.1016/j.ymthe.2021.01.030.
- ↑ "An Open Label Phase II Randomized Trial of BNT113 in Combination With Pembrolizumab Versus Pembrolizumab Monotherapy as a First Line Therapy in Patients With Unresectable Recurrent, or Metastatic Head and Neck Squamous Cell Carcinoma (HNSCC) Which is Positive for Human Papilloma Virus 16 (HPV16+) and Expresses PD-L1 (AHEAD-MERIT)". clinicaltrials.gov. clinicaltrials.gov. 24 January 2022. Retrieved 21 February 2022.
- ↑ Welle (www.dw.com), Deutsche. "COVID vaccine: EU orders 300 million more BioNTech-Pfizer doses | DW | 08.01.2021". DW.COM. Retrieved 13 January 2022.
- ↑ Krienke, Christina; Kolb, Laura; Diken, Elif; Streuber, Michael; Kirchhoff, Sarah; Bukur, Thomas; Akilli-Öztürk, Özlem; Kranz, Lena M.; Berger, Hendrik; Petschenka, Jutta; Diken, Mustafa; Kreiter, Sebastian; Yogev, Nir; Waisman, Ari; Karikó, Katalin; Türeci, Özlem; Sahin, Ugur (8 January 2021). "A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis". Science. doi:10.1126/science.aay3638.
- ↑ "Phase I/IIa, First-in-human, Open-label, Dose Escalation Trial With Expansion Cohorts to Evaluate Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy of BNT151 as a Monotherapy and in Combination With Other Anti-cancer Agents in Patients With Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 30 December 2021. Retrieved 21 February 2022.
- ↑ Vogel, Annette B.; Kanevsky, Isis; Che, Ye; Swanson, Kena A.; Muik, Alexander; Vormehr, Mathias; Kranz, Lena M.; Walzer, Kerstin C.; Hein, Stephanie; Güler, Alptekin; Loschko, Jakob; Maddur, Mohan S.; Ota-Setlik, Ayuko; Tompkins, Kristin; Cole, Journey; Lui, Bonny G.; Ziegenhals, Thomas; Plaschke, Arianne; Eisel, David; Dany, Sarah C.; Fesser, Stephanie; Erbar, Stephanie; Bates, Ferdia; Schneider, Diana; Jesionek, Bernadette; Sänger, Bianca; Wallisch, Ann-Kathrin; Feuchter, Yvonne; Junginger, Hanna; Krumm, Stefanie A.; Heinen, André P.; Adams-Quack, Petra; Schlereth, Julia; Schille, Stefan; Kröner, Christoph; de la Caridad Güimil Garcia, Ramón; Hiller, Thomas; Fischer, Leyla; Sellers, Rani S.; Choudhary, Shambhunath; Gonzalez, Olga; Vascotto, Fulvia; Gutman, Matthew R.; Fontenot, Jane A.; Hall-Ursone, Shannan; Brasky, Kathleen; Griffor, Matthew C.; Han, Seungil; Su, Andreas A. H.; Lees, Joshua A.; Nedoma, Nicole L.; Mashalidis, Ellene H.; Sahasrabudhe, Parag V.; Tan, Charles Y.; Pavliakova, Danka; Singh, Guy; Fontes-Garfias, Camila; Pride, Michael; Scully, Ingrid L.; Ciolino, Tara; Obregon, Jennifer; Gazi, Michal; Carrion, Ricardo; Alfson, Kendra J.; Kalina, Warren V.; Kaushal, Deepak; Shi, Pei-Yong; Klamp, Thorsten; Rosenbaum, Corinna; Kuhn, Andreas N.; Türeci, Özlem; Dormitzer, Philip R.; Jansen, Kathrin U.; Sahin, Ugur (April 2021). "BNT162b vaccines protect rhesus macaques from SARS-CoV-2". Nature. 592 (7853): 283–289. doi:10.1038/s41586-021-03275-y. ISSN 1476-4687.
- ↑ Xie, Xuping; Liu, Yang; Liu, Jianying; Zhang, Xianwen; Zou, Jing; Fontes-Garfias, Camila R.; Xia, Hongjie; Swanson, Kena A.; Cutler, Mark; Cooper, David; Menachery, Vineet D.; Weaver, Scott C.; Dormitzer, Philip R.; Shi, Pei-Yong (April 2021). "Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera". Nature Medicine. 27 (4): 620–621. doi:10.1038/s41591-021-01270-4. ISSN 1546-170X.
- ↑ "A PHASE 3, RANDOMIZED, OBSERVER-BLIND STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND IMMUNOGENICITY OF MULTIPLE PRODUCTION LOTS AND DOSE LEVELS OF THE VACCINE CANDIDATE BNT162b2 AGAINST COVID-19 IN HEALTHY PARTICIPANTS 12 THROUGH 50 YEARS OF AGE AND THE SAFETY, TOLERABILITY, AND IMMUNOGENICITY OF BNT162b2 RNA-BASED COVID-19 VACCINE CANDIDATES AS A BOOSTER DOSE IN HEALTHY PARTICIPANTS 18 THROUGH 50 YEARS OF AGE". clinicaltrials.gov. clinicaltrials.gov. 4 August 2021. Retrieved 20 February 2022.
- ↑ "A PHASE 2/3, PLACEBO-CONTROLLED, RANDOMIZED, OBSERVER-BLIND STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND IMMUNOGENICITY OF A SARS-COV-2 RNA VACCINE CANDIDATE (BNT162b2) AGAINST COVID-19 IN HEALTHY PREGNANT WOMEN 18 YEARS OF AGE AND OLDER". clinicaltrials.gov. clinicaltrials.gov. 31 January 2022. Retrieved 20 February 2022.
- ↑ "A Multi-site, Open-label, Phase II, Randomized, Controlled Trial to Compare the Efficacy of RO7198457 Versus Watchful Waiting in Resected, Stage II (High Risk) and Stage III Colorectal Cancer Patients Who Are ctDNA Positive Following Resection". clinicaltrials.gov. clinicaltrials.gov. 26 January 2022. Retrieved 21 February 2022.
- ↑ Lopez, Juanita S.; Camidge, Ross; Iafolla, Marco; Rottey, Sylvie; Schuler, Martin; Hellmann, Matthew; Balmanoukian, Ani; Dirix, Luc; Gordon, Michael; Sullivan, Ryan; Henick, Brian S.; Drake, Charles; Wong, Kit; LoRusso, Patricia; Ott, Patrick; Fong, Lawrence; Schiza, Aglaia; Yachnin, Jeffery; Ottensmeier, Christian; Braiteh, Fadi; Bendell, Johanna; Leidner, Rom; Fisher, George; Jerusalem, Guy; Molenaar-Kuijsten, Laura; Schmidt, Marcus; Laurie, Scott A.; Aljumaily, Raid; Rittmeyer, Achim; Gort, Eelke; Melero, Ignacio; Mueller, Lars; Sabado, Rachel; Twomey, Patrick; Huang, Jack; Yadav, Manesh; Zhang, Jingbin; Mueller, Felicitas; Derhovanessian, Evelyna; Sahin, Ugur; Türeci, Özlem; Powles, Thomas (15 August 2020). "Abstract CT301: A phase Ib study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in combination with atezolizumab in patients with locally advanced or metastatic solid tumors". Bioinformatics, Convergence Science, and Systems Biology: CT301 – CT301. doi:10.1158/1538-7445.AM2020-CT301.
- ↑ "PHASE 1, OPEN-LABEL DOSE-FINDING STUDY TO EVALUATE SAFETY, TOLERABILITY, AND IMMUNOGENICITY AND PHASE 2/3 PLACEBO-CONTROLLED, OBSERVER-BLINDED SAFETY, TOLERABILITY, AND IMMUNOGENICITY STUDY OF A SARS-COV-2 RNA VACCINE CANDIDATE AGAINST COVID-19 IN HEALTHY CHILDREN AND YOUNG ADULTS". clinicaltrials.gov. clinicaltrials.gov. 14 January 2022. Retrieved 19 February 2022.
- ↑ "Pfizer-BioNTech announces 100 per cent efficacy in vaccine for young teens". BlackburnNews.com. 31 March 2021. Retrieved 4 February 2022.
- ↑ "A PHASE 3, RANDOMIZED, OBSERVER-BLIND STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND IMMUNOGENICITY OF MULTIPLE FORMULATIONS OF THE VACCINE CANDIDATE BNT162B2 AGAINST COVID 19 IN HEALTHY ADULTS 18 THROUGH 55 YEARS OF AGE". clinicaltrials.gov. clinicaltrials.gov. 17 December 2021. Retrieved 19 February 2022.
- ↑ "89Zr-DFO-HuMab-5B1 (MVT-2163) Imaging in Pancreatic Cancer or Other CA19-9 Positive Malignancies". clinicaltrials.gov. clinicaltrials.gov. 10 May 2021. Retrieved 21 February 2022.
- ↑ "Tumor-Specific Zr-89 Immuno-PET Imaging in a Human Bladder Cancer Model". vivo.weill.cornell.edu. Retrieved 17 March 2022.
- ↑ Torjesen, I (27 May 2021). "Covid-19: Pfizer-BioNTech vaccine is "likely" responsible for deaths of some elderly patients, Norwegian review finds". BMJ (Clinical research ed.). 373: n1372. doi:10.1136/bmj.n1372.
- ↑ "Open-label, Randomized Phase II Trial With BNT111 and Cemiplimab in Combination or as Single Agents in Patients With Anti-PD-1-refractory/Relapsed, Unresectable Stage III or IV Melanoma". clinicaltrials.gov. clinicaltrials.gov. 23 November 2021. Retrieved 21 February 2022.
- ↑ "BioNTech Announces First Patient Dosed in Phase 2 Clinical Trial of mRNA-based BNT111 in Patients with Advanced Melanoma | BioNTech". investors.biontech.de. Retrieved 28 February 2022.
- ↑ "Phase I, First-in-human, Open-label, Dose Escalation Trial to Evaluate Safety, Pharmacokinetics, Pharmacodynamics, and Anti-tumor Activity of BNT152+153 in Patients With Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 8 September 2021. Retrieved 21 February 2022.
- ↑ "Open-label, Dose-escalation Trial to Evaluate the Safety and Pharmacokinetics of GEN1046 in Japanese Subjects With Advanced Solid Malignancies". clinicaltrials.gov. clinicaltrials.gov. 7 January 2022. Retrieved 21 February 2022.
- ↑ Commissioner, Office of the (25 June 2021). "Coronavirus (COVID-19) Update: June 25, 2021". FDA. Retrieved 13 January 2022.
- ↑ "FDA adds warning about rare heart inflammation to Pfizer, Moderna COVID shots". Reuters. 26 June 2021. Retrieved 13 January 2022.
- ↑ "A PHASE 3 MASTER PROTOCOL TO EVALUATE ADDITIONAL DOSE(S) OF BNT162B2 IN HEALTHY INDIVIDUALS PREVIOUSLY VACCINATED WITH BNT162B2". clinicaltrials.gov. clinicaltrials.gov. 26 January 2022. Retrieved 19 February 2022.
- ↑ "Epidemiological Study to Determine the Prevalence of ctDNA Positivity in Participants With Stage II (High Risk) or Stage III CRC After Surgery With Curative (R0) Intent and Subsequent Adjuvant Chemotherapy With Monitoring of ctDNA During Clinical Follow-up". clinicaltrials.gov. clinicaltrials.gov. 31 January 2022. Retrieved 20 February 2022.
- ↑ "BioNTech Announces Deal to Supply Taiwan With 10 Million Coronavirus Vaccines". usnews.com. Retrieved 4 February 2022.
- ↑ Post, The Jakarta. "Drug regulator approves Pfizer-BioNTech vaccine for Indonesia after 50-million-dose deal". The Jakarta Post. Retrieved 4 February 2022.
- ↑ "A Phase II, Open-label, Rollover Trial to Evaluate the Safety and Immunogenicity of One or Two Boosting Doses of Comirnaty or One Dose of BNT162b2s01 in BNT162-01 Trial Subjects, or Two Boosting Doses of Comirnaty in BNT162-04 Trial Subjects". clinicaltrials.gov. clinicaltrials.gov. 12 January 2022. Retrieved 19 February 2022.
- ↑ MD, Matthew S. Davids (20 September 2021). "A Pilot Study of a Personalized Neoantigen Cancer Vaccine With and Without Low-Dose Cyclophosphamide or Pembrolizumab in Treatment Naïve, Asymptomatic Patients With IGHV Unmutated Chronic Lymphocytic Leukemia". clinicaltrials.gov. clinicaltrials.gov. Retrieved 22 February 2022.
- ↑ Research, Center for Biologics Evaluation and (14 December 2021). "Comirnaty". FDA. Retrieved 14 January 2022.
- ↑ Commissioner, Office of the (23 August 2021). "FDA Approves First COVID-19 Vaccine". FDA. Retrieved 14 January 2022.
- ↑ CNN, Jacqueline Howard. "FDA grants full approval to Pfizer/BioNTech Covid-19 vaccine, opening door to more vaccine mandates". CNN. Retrieved 14 January 2022.
{{cite web}}:|last1=has generic name (help) - ↑ "A Phase II Trial to Evaluate the Safety and Immunogenicity of a SARS CoV 2 Multivalent RNA Vaccine in Healthy Subjects". clinicaltrials.gov. clinicaltrials.gov. 16 February 2022. Retrieved 19 February 2022.
- ↑ Hotz, Christian; Wagenaar, Timothy R.; Gieseke, Friederike; Bangari, Dinesh S.; Callahan, Michelle; Cao, Hui; Diekmann, Jan; Diken, Mustafa; Grunwitz, Christian; Hebert, Andy; Hsu, Karl; Bernardo, Marie; Karikó, Katalin; Kreiter, Sebastian; Kuhn, Andreas N.; Levit, Mikhail; Malkova, Natalia; Masciari, Serena; Pollard, Jack; Qu, Hui; Ryan, Sue; Selmi, Abderaouf; Schlereth, Julia; Singh, Kuldeep; Sun, Fangxian; Tillmann, Bodo; Tolstykh, Tatiana; Weber, William; Wicke, Lena; Witzel, Sonja; Yu, Qunyan; Zhang, Yu-An; Zheng, Gang; Lager, Joanne; Nabel, Gary J.; Sahin, Ugur; Wiederschain, Dmitri (8 September 2021). "Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models". Science Translational Medicine. doi:10.1126/scitranslmed.abc7804.
- ↑ "Pfizer, BioNTech submit data for authorisation of COVID-19 vaccine in young children". Nairametrics. 29 September 2021. Retrieved 4 February 2022.
- ↑ "A PHASE 2b, OPEN-LABEL STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND IMMUNOGENICITY OF VACCINE CANDIDATE BNT162b2 IN IMMUNOCOMPROMISED PARTICIPANTS ≥2 YEARS OF AGE". clinicaltrials.gov. clinicaltrials.gov. 31 January 2022. Retrieved 19 February 2022.
- ↑ "A Phase 2, Multicenter, Randomized, Open-Label Trial of GEN1046 as Monotherapy and in Combination With Anti-cancer Therapy in Subjects With Relapsed/Refractory Metastatic Non-Small Cell Lung Cancer After Treatment With Standard of Care Therapy With an Immune Checkpoint Inhibitor". clinicaltrials.gov. clinicaltrials.gov. 26 January 2022. Retrieved 21 February 2022.
- ↑ Welle (www.dw.com), Deutsche. "COVID: Germany recommends only BioNTech-Pfizer shot to under 30s | DW | 10.11.2021". DW.COM. Retrieved 13 January 2022.
- ↑ "BioNTech Reaches Agreement with Senegal, Rwanda to Manufacture mRNA Vaccines in Africa". energycapitalpower.com. Retrieved 13 January 2022.
- ↑ "BioNTech Announces Plans to Manufacture Vaccines in Rwanda and Senegal". KT PRESS. 27 August 2021. Retrieved 4 February 2022.
- ↑ "BioNTech to Construct Manufacturing Facility in Africa". manufacturingglobal.com. 27 October 2021. Retrieved 4 February 2022.
- ↑ "BioNTech to build mRNA vaccine facility in Africa next year". devex.com. Retrieved 13 February 2022.
- ↑ "Full nucleotide base sequences for all of the COVID vaccines approved by the MRHA - a Freedom of Information request to Medicines and Healthcare Products Regulatory Agency". WhatDoTheyKnow. 14 July 2021. Retrieved 14 January 2022.
- ↑ "Sequences of Covid-19 vaccines released via WhatDoTheyKnow". mySociety. 2 November 2021. Retrieved 14 January 2022.
- ↑ "COVID-19: Biontech announces development of adapted vaccine for Omicron variant". Vanguard News. 29 November 2021. Retrieved 4 February 2022.
- ↑ "Ugur Sahin, PDG de BioNTech : « Après la troisième injection, notre vaccin semble fournir une protection de 70 % ou 75 % » contre le Covid-19". Le Monde.fr (in français). 20 December 2021. Retrieved 13 January 2022.
- ↑ Archive, View Author; feed, Get author RSS (20 December 2021). "BioNTech CEO says vaccines alone not enough to fight Omicron". New York Post. Retrieved 13 January 2022.
{{cite web}}:|first1=has generic name (help) - ↑ "UK regulator approves use of Pfizer/BioNTech vaccine in 5 to 11-year olds". GOV.UK. Retrieved 13 January 2022.
- ↑ News, Taiwan (29 November 2021). "Taiwan approves 2nd-dose BioNTech vaccinations for ages 12-17, 3rd dose for adults | Taiwan News | 2021-11-29 10:23:00". Taiwan News. Retrieved 13 January 2022.
{{cite web}}:|last1=has generic name (help) - ↑ "Hong Kong panel recommends booster for fully vaccinated BioNTech users". South China Morning Post. 23 December 2021. Retrieved 13 January 2022.
- ↑ Roy, Mrinalika (5 January 2022). "Pfizer, BioNTech to develop shingles shot, after COVID-19 vaccine success". Reuters. Retrieved 13 January 2022.
- ↑ CNN, Katherine Dillinger. "CDC recommends Pfizer/BioNTech Covid-19 vaccine boosters for children as young as 12". CNN. Retrieved 13 January 2022.
{{cite web}}:|last1=has generic name (help) - ↑ "Comparing the Moderna and Pfizer-Biontech vaccines against SARS-CoV-2 infection". News-Medical.net. 12 January 2022. Retrieved 13 January 2022.
- ↑ Ioannou, George; Locke, Emily; Green, Pamela; Berry, Kristin (7 January 2022). "Comparison of Moderna Versus Pfizer-Biontech COVID-19 Vaccine Outcomes: A Target Trial Emulation Study In the U.S. Veterans Affairs Healthcare System". Social Science Research Network.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "BioNTech and Crescendo Biologics team up to develop multi-specific precision immunotherapies". biopharma-reporter.com. Retrieved 13 January 2022.
- ↑ "Selecta turns to synbio player for $1.1B capsid deal; BioNTech signs $790M immunotherapy deal". Endpoints News. Retrieved 13 January 2022.
- ↑ "BioNTech and Crescendo Biologics Announce Global Collaboration to Develop Multi-specific Precision Immunotherapies | BioNTech". investors.biontech.de. Retrieved 13 January 2022.
- ↑ Ali, Shirin (11 January 2022). "BioNTech has developed a method to predict new high-risk variants of COVID-19". TheHill. Retrieved 13 January 2022.
- ↑ "BioNTech and InstaDeep Developed and Successfully Tested Early Warning System to Detect Potential High-Risk SARS-CoV-2 Variants | BioNTech". investors.biontech.de. Retrieved 13 January 2022.
- ↑ "BioNTech, InstaDeep Develop Early Warning System To Detect High-risk Covid-19 Variants In Advance". nasdaq.com. Retrieved 13 January 2022.
- ↑ Murgia, Madhumita; Barnes, Oliver (12 January 2022). "BioNTech and AI start-up develop tool to predict high-risk coronavirus variants". Financial Times. Retrieved 13 January 2022.
- ↑ "Bloomberg - Are you a robot?". www.bloomberg.com. Retrieved 13 January 2022.
{{cite web}}: Cite uses generic title (help) - ↑ "Advisory Panel on COVID-19 Vaccines convenes meeting to conduct continuous benefit-risk analysis of authorised COVID-19 vaccines". www.info.gov.hk. Retrieved 15 February 2022.
- ↑ "Phase I/IIa, First-in-human, Open-label, Dose Escalation Trial With Expansion Cohorts to Evaluate Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy of BNT141 as a Monotherapy and in Combination With Other Anti-cancer Agents in Patients With CLDN18.2-positive Solid Tumors". clinicaltrials.gov. clinicaltrials.gov. 31 January 2022. Retrieved 21 February 2022.
- ↑ "LuCa-MERIT-1: First-in-human, Open Label, Phase I Dose Confirmation Trial Evaluating the Safety, Tolerability and Preliminary Efficacy of BNT116 Alone and in Combinations in Patients With Advanced Non-small Cell Lung Cancer". clinicaltrials.gov. clinicaltrials.gov. 24 November 2021. Retrieved 19 February 2022.
- ↑ "BioNTech SE". Google Trends. Retrieved 14 January 2022.
- ↑ "BioNTech SE - ADR". www.google.com. Retrieved 14 January 2022.