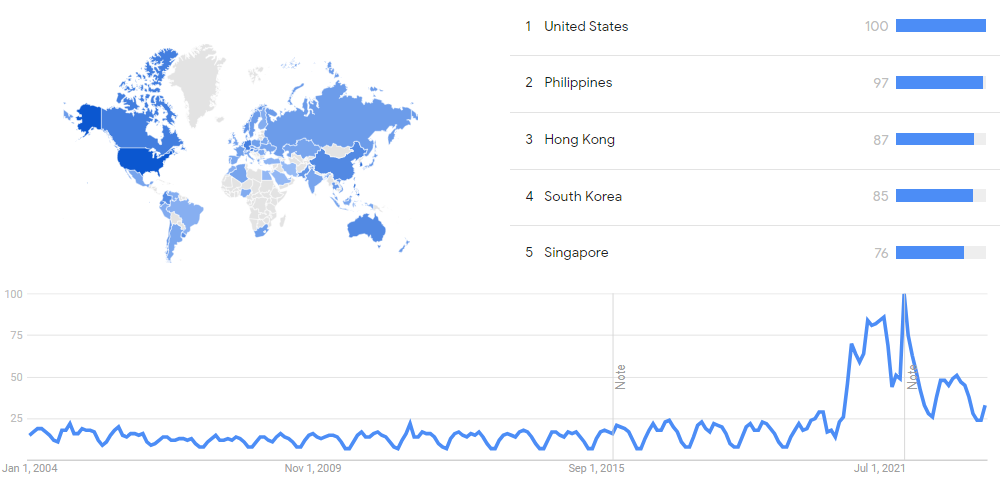
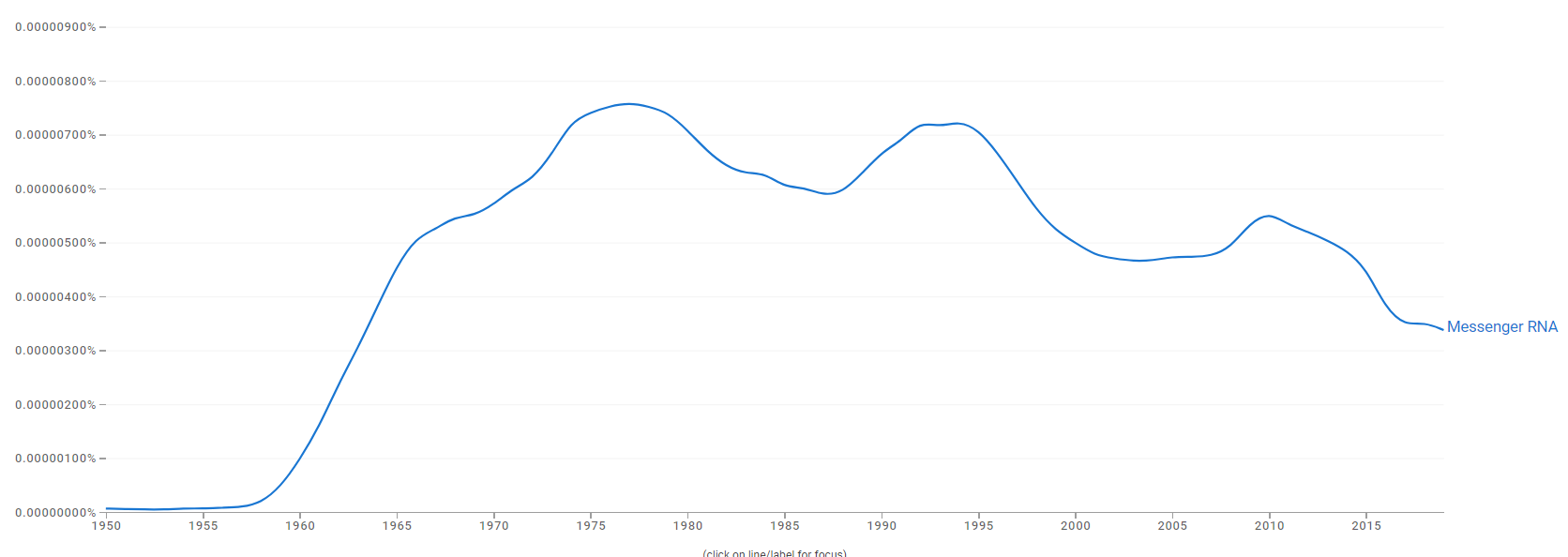
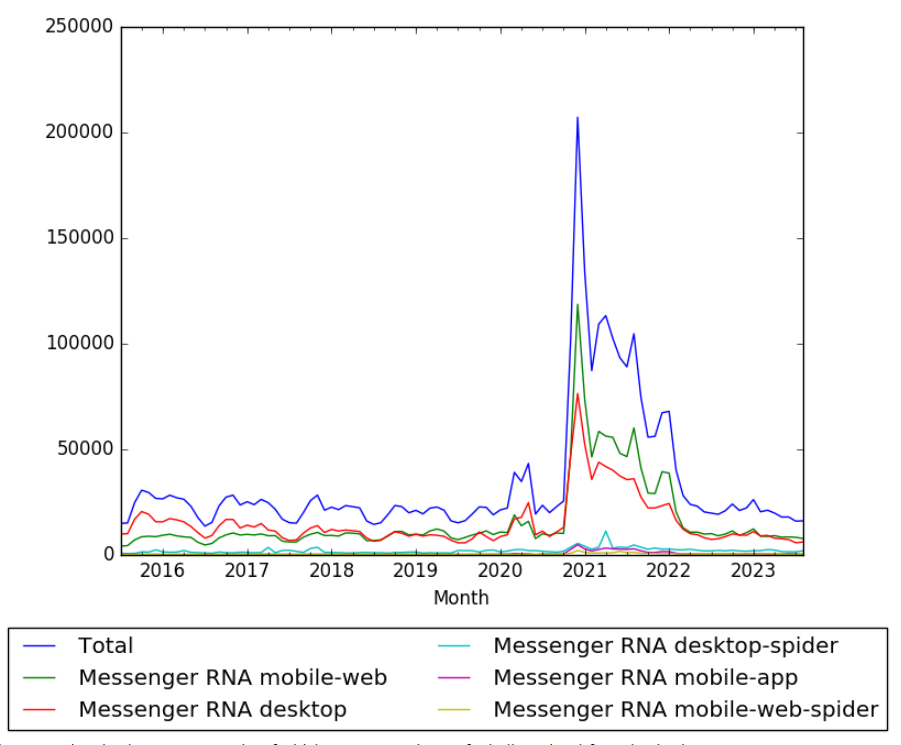
Difference between revisions of "Timeline of mRNA research"
(→What the timeline is still missing) |
(→Sample questions) |
||
| (92 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
The following are some interesting questions that can be answered by reading this timeline: | The following are some interesting questions that can be answered by reading this timeline: | ||
| + | * What are the important discoveries that contributed to the identification and understanding of mRNA? | ||
| + | ** Sort the full timeline by "Event type" and look for the group of rows with value "Discovery". | ||
| + | ** You will see a chronology of significant scientific discoveries related to mRNA research, highlighting key milestones, influential experiments, and important figures in the field. | ||
| + | * What are some milestone experiments in mRNA research that contributed to modern medicine? | ||
| + | ** Sort the full timeline by "Event type" and look for the group of rows with value "Experiment". | ||
| + | ** You will receive a chronological list of key experiments related to mRNA research. These experiments highlight significant advancements in understanding mRNA's role in gene expression, protein synthesis, and its therapeutic potential, including applications in cancer immunotherapy, cardiovascular treatments, gene therapy, and vaccine development. | ||
| + | * What are some key hypotheses that contributed to the understanding of mRNA? | ||
| + | ** Sort the full timeline by "Event type" and look for the group of rows with value "Hypothesis". | ||
| + | ** You will see a chronological list of key hypotheses related to mRNA research. This list includes significant scientific propositions that contributed to the understanding of mRNA's role in gene expression, protein synthesis, and potential applications, highlighting important developments in molecular biology over the past century. | ||
| + | * What are the key technological advancements in mRNA research? | ||
| + | ** Sort the full timeline by "Event type" and look for the group of rows with value "Technological advancement". | ||
| + | ** You'll see a chronological list of key technological advancements in mRNA research, highlighting breakthroughs in mRNA delivery systems, gene therapy, and biotechnology. | ||
| + | * Other events are described under the following types: "Background". | ||
==Big picture== | ==Big picture== | ||
| Line 23: | Line 36: | ||
{| class="sortable wikitable" | {| class="sortable wikitable" | ||
| − | ! Year !! | + | ! Year !! Condition (when applicable) !! Event type !! Details !! Location |
|- | |- | ||
| − | | 1909 || || || German physician {{w|Paul Ehrlich}} suggest that the immune system may suppress tumor development. Today this may be demonstrated through promising applications for synthetic mRNA is immunotherapy for cancer<ref name="Rhoads"/> || {{w|Germany}} | + | | 1909 || {{w|Cancer}} || Hypothesis || German physician {{w|Paul Ehrlich}} suggest that the immune system may suppress tumor development. Today this may be demonstrated through promising applications for synthetic mRNA is immunotherapy for cancer<ref name="Rhoads"/> || {{w|Germany}} |
|- | |- | ||
| − | | 1944 || || || A significant milestone is reached with the publication of {{w|Oswald Avery}}, {{w|Colin Munro MacLeod}}, and {{w|Maclyn McCarty}}'s seminal paper. This influential work identifies the "transforming principle" within pneumococcal bacteria as DNA. The publication marks a pivotal moment in establishing the recognition of DNA's genetic role, firmly establishing it as the carrier of genetic information and its fundamental importance in biological processes and inheritance.<ref name="Cobb"/><ref>{{cite journal |last1=Avery |first1=O. T. |last2=Macleod |first2=C. M. |last3=McCarty |first3=M. |title=STUDIES ON THE CHEMICAL NATURE OF THE SUBSTANCE INDUCING TRANSFORMATION OF PNEUMOCOCCAL TYPES : INDUCTION OF TRANSFORMATION BY A DESOXYRIBONUCLEIC ACID FRACTION ISOLATED FROM PNEUMOCOCCUS TYPE III |journal=The Journal of Experimental Medicine |date=1 February 1944 |volume=79 |issue=2 |pages=137–158 |doi=10.1084/jem.79.2.137 |url=https://pubmed.ncbi.nlm.nih.gov/19871359/ |issn=0022-1007}}</ref><ref>{{cite journal |last1=Cobb |first1=Matthew |title=Oswald Avery, DNA, and the transformation of biology |journal=Current Biology |date=January 2014 |volume=24 |issue=2 |pages=R55–R60 |doi=10.1016/j.cub.2013.11.060}}</ref> || {{w|United States}} ({{w|Rockefeller University}})<ref>{{cite journal |last1=Cobb |first1=Matthew |title=Oswald Avery, DNA, and the transformation of biology |journal=Current Biology |date=January 2014 |volume=24 |issue=2 |pages=R55–R60 |doi=10.1016/j.cub.2013.11.060}}</ref> | + | | 1944 || ''{{w|Streptococcus pneumoniae}}'' || Discovery || A significant milestone is reached with the publication of {{w|Oswald Avery}}, {{w|Colin Munro MacLeod}}, and {{w|Maclyn McCarty}}'s seminal paper. This influential work identifies the "transforming principle" within pneumococcal bacteria as DNA. The publication marks a pivotal moment in establishing the recognition of DNA's genetic role, firmly establishing it as the carrier of genetic information and its fundamental importance in biological processes and inheritance.<ref name="Cobb"/><ref>{{cite journal |last1=Avery |first1=O. T. |last2=Macleod |first2=C. M. |last3=McCarty |first3=M. |title=STUDIES ON THE CHEMICAL NATURE OF THE SUBSTANCE INDUCING TRANSFORMATION OF PNEUMOCOCCAL TYPES : INDUCTION OF TRANSFORMATION BY A DESOXYRIBONUCLEIC ACID FRACTION ISOLATED FROM PNEUMOCOCCUS TYPE III |journal=The Journal of Experimental Medicine |date=1 February 1944 |volume=79 |issue=2 |pages=137–158 |doi=10.1084/jem.79.2.137 |url=https://pubmed.ncbi.nlm.nih.gov/19871359/ |issn=0022-1007}}</ref><ref>{{cite journal |last1=Cobb |first1=Matthew |title=Oswald Avery, DNA, and the transformation of biology |journal=Current Biology |date=January 2014 |volume=24 |issue=2 |pages=R55–R60 |doi=10.1016/j.cub.2013.11.060}}</ref> || {{w|United States}} ({{w|Rockefeller University}})<ref>{{cite journal |last1=Cobb |first1=Matthew |title=Oswald Avery, DNA, and the transformation of biology |journal=Current Biology |date=January 2014 |volume=24 |issue=2 |pages=R55–R60 |doi=10.1016/j.cub.2013.11.060}}</ref> |
|- | |- | ||
| − | | 1947 || || || French biochemist André Boivin becomes the first person to propose the concept that DNA is responsible for generating RNA, which subsequently leads to protein synthesis. Boivin's pioneering insight would lay the foundation for the understanding of the central dogma of {{w|molecular biology}}, highlighting the flow of genetic information from DNA to RNA to proteins.<ref name="Cobb"/> || {{w|France}} | + | | 1947 || || Hypothesis || French biochemist André Boivin becomes the first person to propose the concept that DNA is responsible for generating RNA, which subsequently leads to protein synthesis. Boivin's pioneering insight would lay the foundation for the understanding of the central dogma of {{w|molecular biology}}, highlighting the flow of genetic information from DNA to RNA to proteins.<ref name="Cobb"/> || {{w|France}} |
|- | |- | ||
| − | | 1950 || | + | | 1950 (March 1) || || Hypothesis || Jeener and Szafarz of the {{w|University of Brussels}} first hypothesize that {{w|RNA}} is synthesized in the {{w|cell nucleus}} and then transferred into the {{w|cytoplasm}}, where it was aggregating with other molecules.<ref name="Oeffinger">{{cite book |last1=Oeffinger |first1=Marlene |last2=Zenklusen |first2=Daniel |title=The Biology of mRNA: Structure and Function |date=6 December 2019 |publisher=Springer Nature |isbn=978-3-030-31434-7 |url=https://books.google.com.ar/books/about/The_Biology_of_MRNA_Structure_and_Functi.html?id=9T7CDwAAQBAJ&source=kp_book_description&redir_esc=y |language=en}}</ref><ref>{{cite journal |last1=Jeener |first1=R. |last2=Szafarz |first2=D. |title=Relations Between the Rate of Renewal and the Intracellular Localization of Ribonucleic Acid |journal=Arch. Biochem. |date=1 March 1950 |volume=Vol: 26 |url=https://www.osti.gov/biblio/4402381 |language=English}}</ref> "The first suggestion that small RNA molecules move from the nucleus to the cytoplasm and associate with ribosomes where they drive protein synthesis was made by Raymond Jeener, in 1950."<ref name="Cobb"/> || {{w|Belgium}} |
|- | |- | ||
| − | | 1952 || | + | | 1952 (September 1) || || Hypothesis || American biochemist {{w|Alexander Dounce}}, of {{w|Rochester Medical School}}, proposes a biochemical model of how protein synthesis occurs on an RNA molecule, not on DNA.<ref>{{cite journal |last1=DOUNCE |first1=AL |title=[Duplicating mechanism for peptide chain and nucleic acid synthesis]. |journal=Enzymologia |date=1 September 1952 |volume=15 |issue=5 |pages=251-8 |pmid=13033864}}</ref> Although the model is wrong, Dounce hypothesises that “the specific arrangement of amino acid residues in a given peptide chain is derived from the specific arrangement of nucleotide residues in a corresponding specific nucleic acid molecule”.<ref name="Cobb"/> || {{w|United States}} |
|- | |- | ||
| − | | 1952–1954 || || || French biochemist {{w|Jacques Monod}}'s research team and later American biochemist {{w|Arthur Pardee}} conduct experiments that reveal an interesting connection between RNA synthesis and protein synthesis in mutant bacteria. Their findings show that the production of β-galactosidase, an enzyme, was dependent on the presence of a specific RNA nucleotide called uracil. This discovery suggests that RNA synthesis plays a vital role in the process of protein synthesis. The interpretation shared by Crick and the researchers is that the presence of uracil indicates turnover of RNA molecules in the cytoplasm, implying that some RNA molecules undergo degradation and replacement.<ref>{{cite journal |last1=Monod |first1=J |last2=Pappenheimer |first2=A.M |last3=Cohen-Bazire |first3=G |title=La cinétique de la biosynthèse de la β-galactosidase chez E. coli considérée comme fonction de la croissance |journal=Biochimica et Biophysica Acta |date=January 1952 |volume=9 |pages=648–660 |doi=10.1016/0006-3002(52)90227-8}}</ref><ref>{{cite journal |last1=Pardee |first1=Arthur B. |title=NUCLEIC ACID PRECURSORS AND PROTEIN SYNTHESIS |journal=Proceedings of the National Academy of Sciences |date=May 1954 |volume=40 |issue=5 |pages=263–270 |doi=10.1073/pnas.40.5.263}}</ref><ref name="Cobb"/> || | + | | 1952–1954 || || Discovery || French biochemist {{w|Jacques Monod}}'s research team and later American biochemist {{w|Arthur Pardee}} conduct experiments that reveal an interesting connection between RNA synthesis and protein synthesis in mutant bacteria. Their findings show that the production of β-galactosidase, an enzyme, was dependent on the presence of a specific RNA nucleotide called uracil. This discovery suggests that RNA synthesis plays a vital role in the process of protein synthesis. The interpretation shared by Crick and the researchers is that the presence of uracil indicates turnover of RNA molecules in the cytoplasm, implying that some RNA molecules undergo degradation and replacement.<ref>{{cite journal |last1=Monod |first1=J |last2=Pappenheimer |first2=A.M |last3=Cohen-Bazire |first3=G |title=La cinétique de la biosynthèse de la β-galactosidase chez E. coli considérée comme fonction de la croissance |journal=Biochimica et Biophysica Acta |date=January 1952 |volume=9 |pages=648–660 |doi=10.1016/0006-3002(52)90227-8}}</ref><ref>{{cite journal |last1=Pardee |first1=Arthur B. |title=NUCLEIC ACID PRECURSORS AND PROTEIN SYNTHESIS |journal=Proceedings of the National Academy of Sciences |date=May 1954 |volume=40 |issue=5 |pages=263–270 |doi=10.1073/pnas.40.5.263}}</ref><ref name="Cobb"/> || |
|- | |- | ||
| − | | 1953 || || || James Watson and Francis Crick propose the model of the DNA double helix. After this, a new question would arise in the scientific community: how is information encoded by the DNA and how is it translated?<ref name="The DNA Unive">{{cite web |last1=October 29th |first1=reas Ebertz 15 April 2021 |title=The history of mRNA applications |url=https://the-dna-universe.com/2021/04/15/the-history-of-mrna-applications/ |website=The DNA Universe BLOG |access-date=12 March 2022 |date=15 April 2021}}</ref> The mRNA molecule would be originally discovered as a result of scientists' search to understand the molecular mechanism by which DNA directs the formation of proteins within a cell, a search that would begin as soon as DNA was cracked by Watson and Crick.<ref name="WhatisBiote"/> || {{w|United Kingdom}} | + | | 1953 || || Discovery || James Watson and Francis Crick propose the model of the DNA double helix. After this, a new question would arise in the scientific community: how is information encoded by the DNA and how is it translated?<ref name="The DNA Unive">{{cite web |last1=October 29th |first1=reas Ebertz 15 April 2021 |title=The history of mRNA applications |url=https://the-dna-universe.com/2021/04/15/the-history-of-mrna-applications/ |website=The DNA Universe BLOG |access-date=12 March 2022 |date=15 April 2021}}</ref> The mRNA molecule would be originally discovered as a result of scientists' search to understand the molecular mechanism by which DNA directs the formation of proteins within a cell, a search that would begin as soon as DNA was cracked by Watson and Crick.<ref name="WhatisBiote"/> || {{w|United Kingdom}} |
|- | |- | ||
| − | | 1953 || || || Al Hershey and his research team demonstrate that following phage infection, bacteria exhibits a significant increase in the production of a specific type of RNA, which is synthesized at a high rate but also degraded quickly. However, it remains unclear whether this phenomenon is a result of the infection itself and could be considered a pathological consequence.<ref name="Cobb"/><ref>{{cite journal |last1=Hershey |first1=A. D. |last2=Dixon |first2=June |last3=Chase |first3=Martha |title=NUCLEIC ACID ECONOMY IN BACTERIA INFECTED WITH BACTERIOPHAGE T2 |journal=Journal of General Physiology |date=20 July 1953 |volume=36 |issue=6 |pages=777–789 |doi=10.1085/jgp.36.6.777}}</ref> || | + | | 1953 || || Discovery || Al Hershey and his research team demonstrate that following phage infection, bacteria exhibits a significant increase in the production of a specific type of RNA, which is synthesized at a high rate but also degraded quickly. However, it remains unclear whether this phenomenon is a result of the infection itself and could be considered a pathological consequence.<ref name="Cobb"/><ref>{{cite journal |last1=Hershey |first1=A. D. |last2=Dixon |first2=June |last3=Chase |first3=Martha |title=NUCLEIC ACID ECONOMY IN BACTERIA INFECTED WITH BACTERIOPHAGE T2 |journal=Journal of General Physiology |date=20 July 1953 |volume=36 |issue=6 |pages=777–789 |doi=10.1085/jgp.36.6.777}}</ref> || |
|- | |- | ||
| − | | 1956 || || || Elliot Volkin and Lazarus Astrachan at {{w|Oak Ridge National Laboratory}} discover a 'DNA-like RNA' substance that does not resemble previously found types of RNA. Volkin and Astrachan notice it after infecting ''{{w|Escherichia coli}}'' with {{w|Enterobacteria phage T2}} and then exposing the culture to radioactive {{w|Phosphorus-32}} for a few minutes. Importantly the substance would appear to help the bacteria's cell machinery switch from making its own proteins to those that were characteristic of the virus.<ref name="WhatisBiote"/><ref name="Cobb">{{cite journal |last1=Cobb |first1=Matthew |title=Who discovered messenger RNA? |journal=Current Biology |date=June 2015 |volume=25 |issue=13 |pages=R526–R532 |doi=10.1016/j.cub.2015.05.032 |access-date=16 May 2022}}</ref> || {{w|United States}} | + | | 1956 || ''{{w|Escherichia coli}}'' || Discovery || Elliot Volkin and Lazarus Astrachan at {{w|Oak Ridge National Laboratory}} discover a 'DNA-like RNA' substance that does not resemble previously found types of RNA. Volkin and Astrachan notice it after infecting ''{{w|Escherichia coli}}'' with {{w|Enterobacteria phage T2}} and then exposing the culture to radioactive {{w|Phosphorus-32}} for a few minutes. Importantly the substance would appear to help the bacteria's cell machinery switch from making its own proteins to those that were characteristic of the virus.<ref name="WhatisBiote"/><ref name="Cobb">{{cite journal |last1=Cobb |first1=Matthew |title=Who discovered messenger RNA? |journal=Current Biology |date=June 2015 |volume=25 |issue=13 |pages=R526–R532 |doi=10.1016/j.cub.2015.05.032 |access-date=16 May 2022}}</ref> || {{w|United States}} |
|- | |- | ||
| − | | 1957 || || || The recognition that genes generate a messenger molecule is first realized in Paris when Arthur Pardee visits the Institut Pasteur during his sabbatical. This pivotal observation marks a significant turning point in molecular biology, as it reveals the concept of gene expression through the production of a messenger molecule<ref>{{cite journal |last1=Pardee |first1=Arthur B. |title=PaJaMas in Paris |journal=Trends in Genetics |date=November 2002 |volume=18 |issue=11 |pages=585–587 |doi=10.1016/S0168-9525(02)02780-4}}</ref><ref name="Cobb"/><ref>{{cite web |title=Some historical remarks on Jacob-Monod operon model |url=https://hal.science/hal-03741817/document |website=hal.science |access-date=2 June 2023}}</ref> || {{w|France}} ({{w|Pasteur Institute}}) | + | | 1957 || || Discovery || The recognition that genes generate a messenger molecule is first realized in Paris when Arthur Pardee visits the Institut Pasteur during his sabbatical. This pivotal observation marks a significant turning point in molecular biology, as it reveals the concept of gene expression through the production of a messenger molecule<ref>{{cite journal |last1=Pardee |first1=Arthur B. |title=PaJaMas in Paris |journal=Trends in Genetics |date=November 2002 |volume=18 |issue=11 |pages=585–587 |doi=10.1016/S0168-9525(02)02780-4}}</ref><ref name="Cobb"/><ref>{{cite web |title=Some historical remarks on Jacob-Monod operon model |url=https://hal.science/hal-03741817/document |website=hal.science |access-date=2 June 2023}}</ref> || {{w|France}} ({{w|Pasteur Institute}}) |
|- | |- | ||
| − | | 1958 || || || RNA-rich microsomal particles are baptised "{{w|ribosome}}s", during informal discussions at a conference.<ref name="Cobb"/> || | + | | 1958 || || Nomenclature || RNA-rich microsomal particles are baptised "{{w|ribosome}}s", during informal discussions at a conference. This term is coined to describe these cellular structures, which play a crucial role in protein synthesis. Ribosomes are composed of ribosomal RNA (rRNA) and proteins, and they function as the sites where mRNA is translated into proteins. The naming and subsequent research into ribosomes significantly advances our understanding of cellular processes and the molecular machinery involved in translating genetic information into functional proteins. This breakthrough lays the foundation for further studies in molecular biology and genetics.<ref name="Cobb"/> || |
|- | |- | ||
| − | | 1958 || | + | | 1958 (September 29) || || Experiment || E. Volkin and L. Astrachan conduct research that reveals important findings about the relationship between RNA and DNA. Their experiments demonstrate that after phage infection in bacteria, radioactive RNA appears rapidly. However, when the radioactive isotope is added later, more radioactivity is observed in DNA compared to RNA. This leads them to speculate about the potential role of RNA as a precursor to DNA synthesis.<ref>{{cite journal |last1=Astrachan |first1=L. |last2=Volkin |first2=E. |title=Properties of ribonucleic acid turnover in T2-infected Escherichia coli |journal=Biochimica et Biophysica Acta |date=September 1958 |volume=29 |issue=3 |pages=536–544 |doi=10.1016/0006-3002(58)90010-6 |url=}}</ref><ref>T.H. Jukes |
Federation meetings ''Nature'', 267 (1977), p. 8</ref><ref name="Cobb"/> || | Federation meetings ''Nature'', 267 (1977), p. 8</ref><ref name="Cobb"/> || | ||
|- | |- | ||
| − | | 1960 || || || The idea of mRNA is first conceived by {{w|Sydney Brenner}} and {{w|Francis Crick}} on 15 April 1960 at {{w|King's College, Cambridge}}, while {{w|François Jacob}} tells them about a recent experiment conducted by {{w|Arthur Pardee}}, himself, and {{w|Jacques Monod}}.<ref name="Cobb"/> || {{w|United Kingdom}} | + | | 1960 || || Hypothesis || The idea of mRNA is first conceived by {{w|Sydney Brenner}} and {{w|Francis Crick}} on 15 April 1960 at {{w|King's College, Cambridge}}, while {{w|François Jacob}} tells them about a recent experiment conducted by {{w|Arthur Pardee}}, himself, and {{w|Jacques Monod}}.<ref name="Cobb"/> || {{w|United Kingdom}} |
|- | |- | ||
| − | | 1960 || || || Masayasu Nomura, Benjamin Hall, and {{w|Sol Spiegelman}} make important progress in the study of RNA synthesis after phage infection. They build upon the previous work of Volkin and Astrachan and identify two distinct forms of RNA: one present in the ribosomal fraction and the other in soluble RNA. The researchers propose two possible interpretations for the soluble RNA fraction. It could be a precursor of ribosomal RNA or its breakdown product, or it might be involved in the amino acid acceptance process similar to Crick's adaptor molecule concept. Their findings shed further light on the intricate mechanisms of RNA function and its role in protein synthesis. <ref>{{cite journal |last1=Nomura |first1=Masayasu |last2=Hall |first2=Benjamin D. |last3=Spiegelman |first3=S. |title=Characterization of RNA synthesized in Escherichia coli after bacteriophage T2 infection |journal=Journal of Molecular Biology |date=November 1960 |volume=2 |issue=5 |pages=306–IN4 |doi=10.1016/S0022-2836(60)80027-7}}</ref><ref name="Cobb"/> || | + | | 1960 || || Discovery || Masayasu Nomura, Benjamin Hall, and {{w|Sol Spiegelman}} make important progress in the study of RNA synthesis after phage infection. They build upon the previous work of Volkin and Astrachan and identify two distinct forms of RNA: one present in the ribosomal fraction and the other in soluble RNA. The researchers propose two possible interpretations for the soluble RNA fraction. It could be a precursor of ribosomal RNA or its breakdown product, or it might be involved in the amino acid acceptance process similar to Crick's adaptor molecule concept. Their findings shed further light on the intricate mechanisms of RNA function and its role in protein synthesis. <ref>{{cite journal |last1=Nomura |first1=Masayasu |last2=Hall |first2=Benjamin D. |last3=Spiegelman |first3=S. |title=Characterization of RNA synthesized in Escherichia coli after bacteriophage T2 infection |journal=Journal of Molecular Biology |date=November 1960 |volume=2 |issue=5 |pages=306–IN4 |doi=10.1016/S0022-2836(60)80027-7}}</ref><ref name="Cobb"/> || |
|- | |- | ||
| − | | 1960 || | + | | 1960 (December) || || Publication || American scientists {{w|Sol Spiegelman}} and {{w|Benjamin Hall}} publish an article showing that in {{w|enterobacteria phage T2}}, DNA and transitory RNA show sequence complementarity and would hybridize.<ref name="Cobb"/><ref>{{cite journal |last1=Hall |first1=Benjamin D. |last2=Spiegelman |first2=S. |title=SEQUENCE COMPLEMENTARITY OF T2-DNA AND T2-SPECIFIC RNA |journal=Proceedings of the National Academy of Sciences |date=February 1961 |volume=47 |issue=2 |pages=137–146 |doi=10.1073/pnas.47.2.137}}</ref> The invention of DNA-RNA hybridization by Hall and Spiegelman would have a powerful impact on the theory and discourse of molecular biology.<ref>{{cite journal |last1=Fisher |first1=Susie |title=Not just “a clever way to detect whether DNA really made RNA”1: The invention of DNA–RNA hybridization and its outcome1Judson (1979, p. 440). |journal=Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences |date=October 2015 |volume=53 |pages=40–52 |doi=10.1016/j.shpsc.2015.07.002}}</ref> || {{w|United States}} |
|- | |- | ||
| − | | 1961 || | + | | 1961 || || Discovery || Aronson and McCarthy describe RNA molecules of similar size that were attached to ribosomes. This discovery contributes to the understanding of messenger RNA (mRNA) as a key player in protein synthesis. By identifying that these RNA molecules are associated with ribosomes, the cellular structures responsible for translating genetic information into proteins, Aronson and McCarthy provide important insights into the role of mRNA in conveying genetic instructions from DNA to the ribosome, where proteins are assembled. This work is foundational in establishing the concept of mRNA as a crucial intermediary in the process of gene expression.<ref>{{cite journal |last1=Aronson |first1=Arthur I. |last2=McCarthy |first2=Brian J. |title=Studies of E. coli Ribosomal RNA and Its Degradation Products |journal=Biophysical Journal |date=January 1961 |volume=1 |issue=3 |pages=215–226 |doi=10.1016/S0006-3495(61)86885-9}}</ref> || |
|- | |- | ||
| − | | 1961 || | + | | 1961 (March) || || Publication || American biochemist and geneticist {{w|Marshall Warren Nirenberg}} and his post-doctoral researcher, {{w|J. Heinrich Matthaei}}, publish an article in the journal ''{{w|Biochemical and Biophysical Research Communications}}''. The article documents their cell-free protein synthesis system, highlighting the requirement for both ribosomal RNA and soluble RNA for successful protein synthesis. They find that soluble RNA alone is insufficient to drive the process. Their attempt to fractionate the ribosomal RNA indicates that the biological activity responsible for protein synthesis was associated with a fraction that sedimented approximately three times faster than soluble RNA. This research would shed light on the critical role of ribosomal RNA in the process of protein synthesis.<ref>{{cite journal |last1=Matthaei |first1=Heinrich |last2=Nirenberg |first2=Marshall W. |title=The dependence of cell-free protein synthesis in E.coli upon RNA prepared from ribosomes |journal=Biochemical and Biophysical Research Communications |date=April 1961 |volume=4 |issue=6 |pages=404–408 |doi=10.1016/0006-291x(61)90298-4}}</ref><ref name="Cobb"/> || |
|- | |- | ||
| − | | 1961 || || || A groundbreaking discovery is made with the isolation and characterization of {{w|messenger RNA}} (mRNA). This discovery, published in two articles in Nature, highlights the role of mRNA as an intermediary molecule in {{w|protein synthesis}}. The researchers, including {{w|Sydney Brenner}}, {{w|François Jacob}}, and {{w|Jim Watson}}, find that mRNA carries genetic information from {{w|DNA}} to {{w|ribosome}}s for {{w|protein production}}. This breakthrough revolutionized the understanding of gene function and laid the foundation for subsequent research in molecular biology.<ref>{{cite journal |last1=Brenner |first1=S. |last2=Jacob |first2=F. |last3=Meselson |first3=M. |title=An Unstable Intermediate Carrying Information from Genes to Ribosomes for Protein Synthesis |journal=Nature |date=May 1961 |volume=190 |issue=4776 |pages=576–581 |doi=10.1038/190576a0}}</ref><ref>{{cite journal |last1=Gros |first1=Francois |last2=Hiatt |first2=H. |last3=Gilbert |first3=Walter |last4=Kurland |first4=C. G. |last5=Risebrough |first5=R. W. |last6=Watson |first6=J. D. |title=Unstable Ribonucleic Acid Revealed by Pulse Labelling of Escherichia Coli |journal=Nature |date=May 1961 |volume=190 |issue=4776 |pages=581–585 |doi=10.1038/190581a0}}</ref><ref name="Oeffinger"/><ref name="The DNA Unive"/><ref name="WhatisBiote"/> || | + | | 1961 || || Discovery || A groundbreaking discovery is made with the isolation and characterization of {{w|messenger RNA}} (mRNA). This discovery, published in two articles in Nature, highlights the role of mRNA as an intermediary molecule in {{w|protein synthesis}}. The researchers, including {{w|Sydney Brenner}}, {{w|François Jacob}}, and {{w|Jim Watson}}, find that mRNA carries genetic information from {{w|DNA}} to {{w|ribosome}}s for {{w|protein production}}. This breakthrough revolutionized the understanding of gene function and laid the foundation for subsequent research in molecular biology.<ref>{{cite journal |last1=Brenner |first1=S. |last2=Jacob |first2=F. |last3=Meselson |first3=M. |title=An Unstable Intermediate Carrying Information from Genes to Ribosomes for Protein Synthesis |journal=Nature |date=May 1961 |volume=190 |issue=4776 |pages=576–581 |doi=10.1038/190576a0}}</ref><ref>{{cite journal |last1=Gros |first1=Francois |last2=Hiatt |first2=H. |last3=Gilbert |first3=Walter |last4=Kurland |first4=C. G. |last5=Risebrough |first5=R. W. |last6=Watson |first6=J. D. |title=Unstable Ribonucleic Acid Revealed by Pulse Labelling of Escherichia Coli |journal=Nature |date=May 1961 |volume=190 |issue=4776 |pages=581–585 |doi=10.1038/190581a0}}</ref><ref name="Oeffinger"/><ref name="The DNA Unive"/><ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | 1961 || || | + | | 1961 || Misinterpretation of gene composition || Publication || R.V. Eck publishes a paper in ''[[w:Nature (journal)|Nature]]'' leaving the door open to the possibility that genes are made of proteins, not DNA. This context is essential for understanding the significance of mRNA's discovery as the intermediary in gene expression, confirming DNA's role over proteins.<ref>{{cite journal |last1=ECK |first1=RV |title=Non-randomness in amino-acid 'alleles'. |journal=Nature |date=23 September 1961 |volume=191 |pages=1284-5 |doi=10.1038/1911284a0 |pmid=13889081}}</ref><ref name="Cobb"/> || |
|- | |- | ||
| − | | 1961 || || || | + | | 1961 || || Discovery || In what would be known as the {{w|Nirenberg and Matthaei experiment}}, {{w|Marshall Warren Nirenberg}} and {{w|J. Heinrich Matthaei}} decipher the first {{w|codon}}, UUU, as coding for the amino acid phenylalanine. This pivotal discovery marks a significant milestone in molecular biology, providing the first insights into the genetic code that translates the information encoded in mRNA into proteins. By using a synthetic RNA composed of uracil nucleotides, they demonstrate how specific sequences of nucleotides correspond to particular amino acids, ultimately leading to the synthesis of proteins. This work lays the foundation for understanding the genetic code and the process of translation, profoundly impacting genetics and biochemistry.<ref>{{cite journal |title=Establishing the Triplet Nature of the Genetic Code |author=Charles Yanofsky |journal=Cell |url=https://www.cell.com/cell/pdf/S0092-8674(07)00253-X.pdf |doi=10.1016/j.cell.2007.02.029 |accessdate=16 September 2024}}</ref><ref>{{cite web |title=Nirenberg Introduction |url=https://history.nih.gov/display/history/Nirenberg+Introduction |website=history.nih.gov |accessdate=21 September 2024}}</ref><ref>{{cite web |title=The Experiment that Cracked the Code of Life |url=https://oligofastx.com/the-experiment-that-cracked-the-code-of-life/ |website=oligofastx.com |accessdate=21 September 2024}}</ref><ref>{{cite web |title=Cracking the Genetic Code: Replicating a Scientific Discovery |url=https://www.scienceinschool.org/article/2016/cracking-genetic-code-replicating-scientific-discovery/ |website=scienceinschool.org |accessdate=21 September 2024}}</ref> || {{w|United States}} ({{w|National Institutes of Health}}) |
|- | |- | ||
| − | | 1963 || || Research || Isaacs et al. demonstrate that viral nucleic acids | + | | 1963 || Viral infection response || Research || Isaacs et al. demonstrate that viral nucleic acids could induce the production of interferon in infected chick, rabbit, and mouse cells. This finding is significant because it shows that the presence of viral nucleic acids—considered foreign by the cell—triggers an immune response, specifically the production of interferon, a protein that plays a crucial role in antiviral defense. The study provides early evidence of how cells recognize and respond to foreign genetic material, laying the foundation for understanding innate immune responses to viral infections. This research also contributes to the broader understanding of how cells detect and react to pathogens at the molecular level.<ref>{{cite journal |last1=Isaacs |first1=Alick |title=Interferon |journal=Advances in Virus Research |date=1964 |volume=10 |pages=1–38 |doi=10.1016/S0065-3527(08)60695-8}}</ref><ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 1965 || || || | + | | 1965 || || Discovery || {{w|Marshall W. Nirenberg}} and {{w|Philip Leder}} complete the genetic code by mapping specific codons to amino acids. This achievement is a critical milestone in molecular biology, providing the foundation for understanding how DNA sequences encode proteins. Their work elucidate the universal language of life, revealing the correspondence between nucleotide triplets (codons) in mRNA and the amino acids they specify during protein synthesis. Nirenberg and Leder's groundbreaking research lay the groundwork for deciphering the genetic information encoded in DNA and paved the way for advancements in genetics, biotechnology, and medicine.<ref>{{cite web |title=The Genetic Code: Deciphering the Blueprint of Life |url=https://www.acs.org/education/whatischemistry/landmarks/geneticcode.html |website=American Chemical Society |accessdate=16 September 2024}}</ref> || {{w|United States}} |
|- | |- | ||
| 1965 || October 14 || Recognition || The {{w|Nobel Prize in Physiology or Medicine}} is awarded to {{w|François Jacob}}, {{w|André Lwoff}} and {{w|Jacques Monod}} from {{w|Pasteur Institute}} for the elucidation of the nature of mRNA.<ref name="WhatisBiote"/> || {{w|France}} | | 1965 || October 14 || Recognition || The {{w|Nobel Prize in Physiology or Medicine}} is awarded to {{w|François Jacob}}, {{w|André Lwoff}} and {{w|Jacques Monod}} from {{w|Pasteur Institute}} for the elucidation of the nature of mRNA.<ref name="WhatisBiote"/> || {{w|France}} | ||
|- | |- | ||
| − | | 1969 || || | + | | 1969 || || Experiment || Raymond Lockard and Jerry Lingrel from the University of Cincinnati manage to isolate mRNA from a rabbit and introducing it into mouse {{w|lymphocyte}}s. This experiment demonstrates that the introduced mRNA could stimulate the production of a specific protein in the lymphocytes. This finding marks a significant step in understanding the role and potential of mRNA in {{w|gene expression}} and {{w|protein synthesis}}.<ref name="The DNA Unive"/><ref name="WhatisBiote"/> || {{w|United States}} ({{w|University of Cincinnati}}) |
|- | |- | ||
| − | | 1970 || || || | + | | 1970 || || Discovery || Significant advancements are made in understanding the role of transfer RNA (tRNA) in protein synthesis. Researchers elucidate how tRNA facilitates translation, a key step in the process of converting genetic information from messenger RNA (mRNA) into proteins. tRNA molecules act as adaptors, bringing specific amino acids to the ribosome, the cellular machinery responsible for protein assembly. This discovery clarifies how genetic instructions are translated into functional proteins and contributes to the broader understanding of molecular biology. The insights gained from studying tRNA would have profound implications for genetics, biochemistry, and medicine. |
|- | |- | ||
| − | | 1973 || || | + | | 1973 || || Discovery || Canadian physician {{w|Ralph M. Steinman}} and {{w|Zanvil A. Cohn}} at {{w|Rockefeller University}} discover {{w|dendritic cell}}s, a type of immune cell found in the bloodstream that helps present {{w|antigen}}s to the immune system to activate the {{w|T cell}} response to destroy it. Many of the mRNA cancer vaccines target dendritic cells.<ref>{{cite web |last1=Chapoval |first1=Svetlana P. |title=Dendritic Cells |url=https://www.intechopen.com/books/7248 |website=intechopen |access-date=16 May 2022 |language=en |date=7 November 2018}}</ref><ref name="WhatisBiote"/> || {{w|United States}} |
|- | |- | ||
| − | | 1974 || || | + | | 1974 || || Discovery || Yasuhiro Furuichi from the National Institute of Genetics reports a key discovery regarding mRNA synthesis, when he finds that during the initial stage of transcription of the double-stranded RNA genome, mRNA synthesis could be activated by a specific nucleotide. This finding sheds light on the mechanisms involved in the transcription process and provides insights into the regulation of mRNA synthesis.<ref name="WhatisBiote"/> || {{w|Japan}} ({{w|National Institute of Genetics}}) |
|- | |- | ||
| − | | 1975 || || | + | | 1975 || || Discovery || Yasuhiro Furuichi and A. J. Shatkin at AGENE Research Institute in {{w|Kamakura}}, {{w|Japan}}, discover a unique 'cap' structure at the tip end of mRNA. Known as the 5' cap, this structure plays a crucial role in mRNA stability, transport, and translation initiation. The discovery of the mRNA cap provides valuable insights into the molecular mechanisms of gene expression and paved the way for further research in the field of molecular biology.<ref>{{cite journal |last1=Furuichi |first1=Yasuhiro |last2=Shatkin |first2=Aaron J |title=Viral and cellular mRNA capping: Past and prospects |journal=Advances in Virus Research |date=2000 |volume=55 |pages=135–184 |doi=10.1016/s0065-3527(00)55003-9}}</ref><ref name="WhatisBiote"/><ref name="WhatisBiote"/> || {{w|Japan}} (AGENE Research Institute) |
|- | |- | ||
| − | | 1977 || || || | + | | 1977 || || Discovery || Richard J. Roberts and Phillip A. Sharp independently discover that genes can be discontinuous, existing as separate segments in DNA. This would lead to the understanding of split genes and RNA splicing, revealing the presence of introns and exons. Introns are non-coding sequences spliced out of RNA before translation, while exons are the coding sequences that form mature RNA. This discovery alters the understanding of gene development during evolution, showing that split genes can generate new genes or proteins and that a single gene can encode multiple proteins.<ref>{{cite web |title=The Nobel Prize in Physiology or Medicine 1993 |url=https://www.nobelprize.org/prizes/medicine/1993/press-release/ |website=NobelPrize.org |accessdate=3 August 2024}}</ref><ref>{{cite web |title=MIT Professor Phillip Sharp wins Nobel Prize in medicine |url=https://news.mit.edu/1993/sharp-1020 |website=MIT News |accessdate=3 August 2024}}</ref><ref>{{cite journal |title=The discovery of split genes and RNA splicing |url=https://www.researchgate.net/publication/7791558_The_discovery_of_split_genes_and_RNA_splicing |journal=ResearchGate |accessdate=3 August 2024}}</ref><ref>{{cite web |title=Discoveries of split genes |url=https://www.animalresearch.info/en/medical-advances/nobel-prizes/discoveries-of-split-genes/ |website=AnimalResearch.info |accessdate=3 August 2024}}</ref> || |
|- | |- | ||
| − | | 1978 || || | + | | 1978 || || Technological advancement || {{w|Liposome}}s are first utilized as a method for delivering mRNA to {{w|eukaryotic cell}}s, marking an early step in the development of mRNA-based technologies. Liposomes, which are small vesicles composed of lipid bilayers, provide a protective environment for mRNA, facilitating its entry into cells without degradation. This approach lays the groundwork for future advancements in mRNA delivery systems. By the end of the 1980s, a significant development would occur with the description and commercialization of DOTMA (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride), a cationic liposome mRNA delivery system. DOTMA would become a widely used tool in molecular biology, enhancing the efficiency and reliability of mRNA delivery for research and therapeutic purposes.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 1978 || | + | | 1978 (August) || || Experiment || Dimitriadis, Ostro, Giacomoni, Lavelle, Paxton, and Dray conduct an important experiment at the National Institute for Medical Research and the University of Illinois. They successfully produce proteins in mouse and human cells through the delivery of mRNA that was packaged in a liposome. This groundbreaking approach demonstrates the potential of using mRNA as a therapeutic tool for protein production, opening new avenues for research and development in the field of molecular medicine.<ref>{{cite journal |last1=Dimitriadis |first1=Giorgos J. |title=Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes |journal=Nature |date=August 1978 |volume=274 |issue=5674 |pages=923–924 |doi=10.1038/274923a0 |url=https://www.nature.com/articles/274923a0 |language=en |issn=1476-4687}}</ref><ref name="WhatisBiote"/><ref>{{cite journal |last1=Ostro |first1=M. J. |last2=Giacomoni |first2=D. |last3=Lavelle |first3=D. |last4=Paxton |first4=W. |last5=Dray |first5=S. |title=Evidence for translation of rabbit globin mRNA after liposome-mediated insertion into a human cell line |journal=Nature |date=31 August 1978 |volume=274 |issue=5674 |pages=921–923 |doi=10.1038/274921a0 |url=https://pubmed.ncbi.nlm.nih.gov/683335/ |issn=0028-0836}}</ref> || {{w|United Kingdom}} ({{w|National Institute for Medical Research}}), {{w|United States}} ({{w|University of Illinois}}) |
|- | |- | ||
| − | | 1978 || || || | + | | 1978 || || Discovery || {{w|Thomas Cech}} and {{w|Sidney Altman}} independently discover {{w|ribozyme}}s, a finding that demonstrates RNA molecules can catalyze chemical reactions, a role previously thought to be exclusive to proteins. This discovery challenges the central dogma of {{w|molecular biology}}, which at the time holds that proteins are the sole enzymes. The understanding of ribozymes provides crucial insights into RNA's versatility, laying the foundation for mRNA research. It opens the possibility that RNA can not only carry genetic information but also play active roles in cellular processes, ultimately leading to the development of mRNA-based therapies and vaccines. The 1989 {{w|Nobel Prize in Chemistry}} would be awarded to Tom Cech and Sidney Altman for their independent discoveries of ribozymes.<ref>{{cite web |title=The Nobel Prize in Chemistry 1989 |url=https://www.nobelprize.org/prizes/chemistry/1989/press-release/ |website=Nobel Prize |accessdate=3 September 2024}}</ref> || {{w|United States}} |
|- | |- | ||
| − | | 1983 || || || Jeffrey et al. report that the distribution of actin mRNA in ascidian oocytes and embryos exhibit an asymmetrical pattern. This finding marks the initial identification of key components in understanding the phenomenon.<ref name="Oeffinger"/> || | + | | 1983 || || Discovery || Jeffrey et al. report that the distribution of actin mRNA in ascidian oocytes and embryos exhibit an asymmetrical pattern. This finding marks the initial identification of key components in understanding the phenomenon.<ref name="Oeffinger"/> || |
|- | |- | ||
| − | | 1983 || || || | + | | 1983 || || Discovery || Researchers make a significant discovery by identifying two key features of mRNA: the 5' cap and the 3' polyadenylation. The 5' cap, a modified guanine nucleotide added to the beginning of the mRNA molecule, and the 3' polyadenylation, a tail of adenine nucleotides added to the end, are found to be essential for mRNA stability and the initiation of translation. These modifications protect mRNA from degradation, assist in the export of mRNA from the nucleus, and are crucial for the efficient initiation of protein synthesis. This discovery is pivotal in understanding the regulation of gene expression at the mRNA level. |
|- | |- | ||
| − | | 1984 || || || Philipp Felgner, working at Syntex Research, manages to synthesize the first cationic (positively charged) lipid. The use of cationic lipids provide a promising approach for delivering therapeutic drugs and genetic material into cells more efficiently. This breakthrough marks an important step forward in the development of novel delivery systems and paves the way for further advancements in drug and gene therapy research.<ref name="WhatisBiote"/> || | + | | 1984 || || Technological advancement || Philipp Felgner, working at Syntex Research, manages to synthesize the first cationic (positively charged) lipid. The use of cationic lipids provide a promising approach for delivering therapeutic drugs and genetic material into cells more efficiently. This breakthrough marks an important step forward in the development of novel delivery systems and paves the way for further advancements in drug and gene therapy research.<ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | 1984 || || || Developmental biologists Paul Krieg and Douglas Melton from Harvard University demonstrate the production of abundant and functional mRNA in the laboratory. They achieve this by utilizing an RNA-synthesis enzyme derived from the vaccinia virus and employing various techniques. Their research would pave the way for the generation of significant quantities of biologically active mRNA, contributing to advancements in the field of molecular biology.<ref>{{cite web |last1=Dolgin |first1=Elie |title=The tangled history of mRNA vaccines |url=https://www.nature.com/articles/d41586-021-02483-w |website=Nature |access-date=16 May 2022 |pages=318–324 |language=en |doi=10.1038/d41586-021-02483-w |date=14 September 2021}}</ref><ref name="The DNA Unive"/> || {{w|United States}} ({{w|Harvard University}}) | + | | 1984 || || Technological advancement || Developmental biologists Paul Krieg and Douglas Melton from Harvard University demonstrate the production of abundant and functional mRNA in the laboratory. They achieve this by utilizing an RNA-synthesis enzyme derived from the vaccinia virus and employing various techniques. Their research would pave the way for the generation of significant quantities of biologically active mRNA, contributing to advancements in the field of molecular biology.<ref>{{cite web |last1=Dolgin |first1=Elie |title=The tangled history of mRNA vaccines |url=https://www.nature.com/articles/d41586-021-02483-w |website=Nature |access-date=16 May 2022 |pages=318–324 |language=en |doi=10.1038/d41586-021-02483-w |date=14 September 2021}}</ref><ref name="The DNA Unive"/> || {{w|United States}} ({{w|Harvard University}}) |
|- | |- | ||
| − | | 1985 || || || S. Tabor and C. C. Richardson develop an upgraded version of the T7 polymerase-promoter complex, allowing for precise regulation of gene expression. This innovative approach remains in use today. These groundbreaking experiments initiated a continuous progression of practical research focused on mRNA delivery and commercialization, leading to significant advancements in the field.<ref>{{cite journal |last1=Tabor |first1=S |last2=Richardson |first2=C C |title=A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. |journal=Proceedings of the National Academy of Sciences |date=February 1985 |volume=82 |issue=4 |pages=1074–1078 |doi=10.1073/pnas.82.4.1074}}</ref><ref name="The DNA Unive"/> || | + | | 1985 || || Technological advancement || S. Tabor and C. C. Richardson develop an upgraded version of the T7 polymerase-promoter complex, allowing for precise regulation of gene expression. This innovative approach remains in use today. These groundbreaking experiments initiated a continuous progression of practical research focused on mRNA delivery and commercialization, leading to significant advancements in the field.<ref>{{cite journal |last1=Tabor |first1=S |last2=Richardson |first2=C C |title=A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. |journal=Proceedings of the National Academy of Sciences |date=February 1985 |volume=82 |issue=4 |pages=1074–1078 |doi=10.1073/pnas.82.4.1074}}</ref><ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 1987 || || || American physician and biochemist {{w|Robert W. Malone}} conducts an experiment that demonstrates the ability to induce cells to produce {{w|luciferase}}, a light-producing protein. He achieves this by injecting the cells with mRNA that he had prepared using the technique developed by Krieg and Melton, combined with cationic lipids provided by Felgner. This experiment, conducted at the Salk Institute for Biological Sciences in collaboration with Felgner and Verma, shows that mRNA encapsulated into liposomes made with cationic lipids could effectively stimulate protein production when injected into mouse cells. This breakthrough provides further evidence for the potential of mRNA-based therapies and marks an important milestone in the development of mRNA delivery systems for protein expression.<ref>{{cite web |last1=MD |first1=Justus R. Hope |title=Inventor of mRNA banned by the New England Journal of Medicine |url=https://www.thedesertreview.com/opinion/columnists/inventor-of-mrna-banned-by-the-new-england-journal-of-medicine/article_4350cf46-27af-11ec-bf58-ab6dcf0152c3.html |website=The Desert Review |access-date=5 March 2022 |language=en}}</ref> || {{w|United States}} ({{w|Salk Institute}}) | + | | 1987 || || Experiment || American physician and biochemist {{w|Robert W. Malone}} conducts an experiment that demonstrates the ability to induce cells to produce {{w|luciferase}}, a light-producing protein. He achieves this by injecting the cells with mRNA that he had prepared using the technique developed by Krieg and Melton, combined with cationic lipids provided by Felgner. This experiment, conducted at the Salk Institute for Biological Sciences in collaboration with Felgner and Verma, shows that mRNA encapsulated into liposomes made with cationic lipids could effectively stimulate protein production when injected into mouse cells. This breakthrough provides further evidence for the potential of mRNA-based therapies and marks an important milestone in the development of mRNA delivery systems for protein expression.<ref>{{cite web |last1=MD |first1=Justus R. Hope |title=Inventor of mRNA banned by the New England Journal of Medicine |url=https://www.thedesertreview.com/opinion/columnists/inventor-of-mrna-banned-by-the-new-england-journal-of-medicine/article_4350cf46-27af-11ec-bf58-ab6dcf0152c3.html |website=The Desert Review |access-date=5 March 2022 |language=en}}</ref> || {{w|United States}} ({{w|Salk Institute}}) |
|- | |- | ||
| − | | 1989 || || || mRNA as a therapeutic is first | + | | 1989 || || Technological advancement || The concept of using mRNA as a therapeutic agent is first proposed following the development of a broadly applicable in vitro transfection technique. This technological advancement enables scientists to introduce mRNA into cells in a controlled laboratory setting, laying the groundwork for mRNA-based therapies. The in vitro transfection method allows for the efficient delivery and expression of mRNA within cells, demonstrating its potential to direct the production of therapeutic proteins. This breakthrough marks a pivotal moment in biotechnology, opening the door to innovative approaches in treating diseases by harnessing the power of mRNA.<ref>{{cite journal | vauthors = Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ | title = Developing mRNA-vaccine technologies | journal = RNA Biology | volume = 9 | issue = 11 | pages = 1319–30 | date = November 2012 | pmid = 23064118 | pmc = 3597572 | doi = 10.4161/rna.22269 }}</ref> || |
|- | |- | ||
| − | | 1989 || || || Dimitriadis | + | | 1989 || || Experiment || Dimitriadis conducts an experiment using {{w|liposome}}s for the delivery of mRNA to {{w|eukaryotic cell}}s. This experiment is a significant step in developing methods to effectively introduce mRNA into cells, a critical challenge for mRNA-based therapies. The use of liposomes—spherical vesicles with lipid bilayers—allows for the encapsulation and protection of mRNA, facilitating its entry into cells. By the end of the following decade, this research would lead to the description and commercialization of DOTMA, a cationic liposome mRNA delivery system. DOTMA represents an important advancement in the field, enabling more efficient and targeted delivery of mRNA for therapeutic purposes.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 1990 || | + | | 1990 (January 1) || || Experiment || An experiment by Kariko et al. at {{w|University of Pennsylvania}} shows that mRNA can be used to get cells to produce protein that inhibits blood clot formation.<ref name="WhatisBiote"/> || {{w|United States}} |
|- | |- | ||
| − | | 1990 || | + | | 1990 (March 23) || || Discovery|| A study reports that when naked mRNA and naked DNA were directly injected into the skeletal muscle of mice, it results in the production of proteins. The research was conducted by Wolff, Malone, Williams, Chong, Acsadi, and Jani from the University of Wisconsin, Salk Institute for Biological Sciences, and Vical.<ref name="WhatisBiote"/><ref>{{cite journal |last1=Wolff |first1=Jon A. |last2=Malone |first2=Robert W. |last3=Williams |first3=Phillip |last4=Chong |first4=Wang |last5=Acsadi |first5=Gyula |last6=Jani |first6=Agnes |last7=Felgner |first7=Philip L. |title=Direct Gene Transfer into Mouse Muscle in Vivo |journal=Science |date=23 March 1990 |volume=247 |issue=4949 |pages=1465–1468 |doi=10.1126/science.1690918}}</ref> || {{w|United States}} ({{w|University of Wisconsin}}, {{w|Salk Institute}}) |
|- | |- | ||
| − | | 1990 || || || Following the initial endeavors in DNA-based {{w|gene therapy}}, the viability of mRNA as a genetic vector is demonstrated. This approach holds theoretical appeal due to the non-integration of mRNA into the genome, its immediate availability for protein translation, and its transient nature that is advantageous for certain applications. Wolff et al., in a notable study, inject naked mRNA encoding chloramphenicol acetyltransferase into the skeletal muscle of mice and successfully observed the expression of specific proteins. This marks the first published account of the effective utilization of in vitro transcribed (IVT) mRNA in animals, where injected reporter gene mRNAs results in detectable {{w|protein production}}.<ref name="Rhoads"/><ref name="Nature Re">{{cite web |last1=Pardi |first1=Norbert |last2=Hogan |first2=Michael J. |last3=Porter |first3=Frederick W. |last4=Weissman |first4=Drew |title=mRNA vaccines — a new era in vaccinology |url=https://www.nature.com/articles/nrd.2017.243 |website=Nature Reviews Drug Discovery |access-date=5 March 2022 |pages=261–279 |language=en |doi=10.1038/nrd.2017.243 |date=April 2018}}</ref><ref>{{cite journal |last1=Wolff |first1=Jon A. |last2=Malone |first2=Robert W. |last3=Williams |first3=Phillip |last4=Chong |first4=Wang |last5=Acsadi |first5=Gyula |last6=Jani |first6=Agnes |last7=Felgner |first7=Philip L. |title=Direct Gene Transfer into Mouse Muscle in Vivo |journal=Science |date=23 March 1990 |volume=247 |issue=4949 |pages=1465–1468 |doi=10.1126/science.1690918}}</ref> || | + | | 1990 || || Discovery || Following the initial endeavors in DNA-based {{w|gene therapy}}, the viability of mRNA as a genetic vector is demonstrated. This approach holds theoretical appeal due to the non-integration of mRNA into the genome, its immediate availability for protein translation, and its transient nature that is advantageous for certain applications. Wolff et al., in a notable study, inject naked mRNA encoding chloramphenicol acetyltransferase into the skeletal muscle of mice and successfully observed the expression of specific proteins. This marks the first published account of the effective utilization of in vitro transcribed (IVT) mRNA in animals, where injected reporter gene mRNAs results in detectable {{w|protein production}}.<ref name="Rhoads"/><ref name="Nature Re">{{cite web |last1=Pardi |first1=Norbert |last2=Hogan |first2=Michael J. |last3=Porter |first3=Frederick W. |last4=Weissman |first4=Drew |title=mRNA vaccines — a new era in vaccinology |url=https://www.nature.com/articles/nrd.2017.243 |website=Nature Reviews Drug Discovery |access-date=5 March 2022 |pages=261–279 |language=en |doi=10.1038/nrd.2017.243 |date=April 2018}}</ref><ref>{{cite journal |last1=Wolff |first1=Jon A. |last2=Malone |first2=Robert W. |last3=Williams |first3=Phillip |last4=Chong |first4=Wang |last5=Acsadi |first5=Gyula |last6=Jani |first6=Agnes |last7=Felgner |first7=Philip L. |title=Direct Gene Transfer into Mouse Muscle in Vivo |journal=Science |date=23 March 1990 |volume=247 |issue=4949 |pages=1465–1468 |doi=10.1126/science.1690918}}</ref> || |
|- | |- | ||
| − | | 1990 || || || The concept of mRNA as a therapeutic agent is established by Wolff J. and colleagues, when they conduct an experiment demonstrating the in-vivo expression of mRNA. The researchers inject naked RNA directly into the muscles of mice, providing evidence of direct gene transfer in living organisms. This study lays the foundation for the use of mRNA as a potential tool for therapeutic applications.<ref name="The DNA Unive"/> || | + | | 1990 || || Discovery || The concept of mRNA as a therapeutic agent is established by Wolff J. and colleagues, when they conduct an experiment demonstrating the in-vivo expression of mRNA. The researchers inject naked RNA directly into the muscles of mice, providing evidence of direct gene transfer in living organisms. This study lays the foundation for the use of mRNA as a potential tool for therapeutic applications.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 1991 || || || mRNA is proposed as | + | | 1991 || Cancer || Therapeutic development || mRNA is proposed as a potential active pharmaceutical ingredient for the treatment of cancer. This idea emerges from the growing understanding of mRNA's role in protein synthesis and its potential to be harnessed for therapeutic purposes. The concept involves using mRNA to instruct cells to produce proteins that can stimulate an immune response against cancer cells or directly target and attack tumor cells. This proposal marks an early recognition of mRNA's potential in cancer therapy, paving the way for subsequent developments in the use of mRNA for targeted treatments and immunotherapies in oncology.<ref name="Dolgin">{{cite web |last1=Dolgin |first1=Elie |title=The tangled history of mRNA vaccines |url=https://www.nature.com/articles/d41586-021-02483-w |website=Nature |access-date=9 March 2022 |pages=318–324 |language=en |doi=10.1038/d41586-021-02483-w |date=14 September 2021}}</ref> || |
|- | |- | ||
| − | | 1992 || | + | | 1992 (February 21) || Diabetes || Therapeutic development || Jirikowski et al. at The Scripps Research Institute Diabetes demonstrate that mRNA encoding for the hormone vasopressin directly injected into the brains of rats could temporarily relieve diabetes for up to 5 days.<ref name="WhatisBiote"/><ref name="The DNA Unive"/><ref>{{cite journal |last1=Jirikowski |first1=Gustav F. |last2=Sanna |first2=Pietro Paolo |last3=Maciejewski-Lenoir |first3=Dominique |last4=Bloom |first4=Floyd E. |title=Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA |journal=Science |date=21 February 1992 |volume=255 |issue=5047 |pages=996–998 |doi=10.1126/science.1546298}}</ref> || {{w|United States}} |
|- | |- | ||
| − | | 1992 || || || A significant milestone is reached in the utilization of mRNA as a genetic vector. Prior to this, the theoretical advantages of mRNA, such as its non-integration into the genome, immediate availability for protein translation, and transient signaling, had been recognized. The practical demonstration of mRNA's capabilities occurrs in 1990 when Wolff et al. inject naked mRNA encoding chloramphenicol acetyl transferase into the skeletal muscle of mice, resulting in the targeted expression of the specific protein. Building upon this success, a subsequent study in 1992 focuses on the treatment of chronic diabetes insipidus in Brattleboro rats. In this experiment, naked, synthetic mRNA encoding arginine vasopressin was injected into the rats' hypothalami, leading to a successful cure of the condition. These findings exemplify the potential of mRNA as a genetic tool, showcasing its ability to deliver therapeutic benefits in gene therapy approaches.<ref name="Rhoads"/> || | + | | 1992 || {{w|Diabetes}} || Therapeutic development || A significant milestone is reached in the utilization of mRNA as a genetic vector. Prior to this, the theoretical advantages of mRNA, such as its non-integration into the genome, immediate availability for protein translation, and transient signaling, had been recognized. The practical demonstration of mRNA's capabilities occurrs in 1990 when Wolff et al. inject naked mRNA encoding chloramphenicol acetyl transferase into the skeletal muscle of mice, resulting in the targeted expression of the specific protein. Building upon this success, a subsequent study in 1992 focuses on the treatment of chronic diabetes insipidus in Brattleboro rats. In this experiment, naked, synthetic mRNA encoding arginine vasopressin was injected into the rats' hypothalami, leading to a successful cure of the condition. These findings exemplify the potential of mRNA as a genetic tool, showcasing its ability to deliver therapeutic benefits in gene therapy approaches.<ref name="Rhoads"/> || |
|- | |- | ||
| − | | 1992 || || | + | | 1992 || {{w|Diabetes}} || Therapeutic development || A study demonstrates that administration of {{w|vasopressin}}-encoding mRNA in the {{w|hypothalamus}} could elicit a physiological response in rats. This research highlights the potential of using mRNA as a therapeutic tool by showing that it could effectively instruct cells to produce a specific protein—in this case, vasopressin, a hormone involved in regulating water balance and blood pressure. This study lays the groundwork for further exploration into mRNA-based therapies, suggesting that mRNA can be used to deliver therapeutic proteins and address various medical conditions, including endocrine disorders and potentially diabetes.<ref name="Nature Re"/><ref>{{cite journal |last1=Jirikowski |first1=Gustav F. |last2=Sanna |first2=Pietro Paolo |last3=Maciejewski-Lenoir |first3=Dominique |last4=Bloom |first4=Floyd E. |title=Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA |journal=Science |date=21 February 1992 |volume=255 |issue=5047 |pages=996–998 |doi=10.1126/science.1546298}}</ref> || |
|- | |- | ||
| − | | 1993 || | + | | 1993 (April 20) || || Publication || Joel G. Belasco and George Brawerman publish the book ''Control of Messenger RNA Stability''. This work provides a comprehensive examination of the mechanisms regulating the stability of mRNA molecules within cells. The book discusses how the degradation of mRNA influences gene expression and cellular responses, emphasizing the role of various regulatory elements and processes in controlling mRNA longevity. This publication is a key resource for understanding the dynamic regulation of mRNA stability, which is crucial for gene expression control and has implications for fields such as molecular biology, genetics, and therapeutic development.<ref>{{cite book |last1=Belasco |first1=Joel G. |last2=Brawerman |first2=George |title=Control of Messenger RNA Stability |date=20 April 1993 |publisher=Elsevier Science |isbn=978-0-12-084782-2 |url=https://books.google.com.ar/books/about/Control_of_Messenger_RNA_Stability.html?id=RwhrAAAAMAAJ&source=kp_book_description&redir_esc=y |language=en}}</ref> || {{w|United States}} ({{w|University of Michigan}}) |
|- | |- | ||
| − | | 1993 || | + | | 1993 (July) || || Vaccine development || The first evidence suggesting that mRNA could serve as a potential method for vaccines occurs when researchers Martinon, Krishnan, Lenzen, Magne, Gomard, and Guillet from INSERM present compelling findings supporting the notion that mRNA could be utilized as a viable approach for vaccine development. This discovery opens up new possibilities for utilizing mRNA as a tool in immunization strategies.<ref name="WhatisBiote"/> || {{w|France}} ({{w|Inserm}}) |
|- | |- | ||
| − | | 1993 || || || Martinon et al. show that subcutaneous injection of liposome-encapsidated mRNA encoding the influenza virus nucleoprotein induces anti-influenza cytotoxic [[w:T cell|T lymphocytes]].<ref name="Rhoads"/> || | + | | 1993 || {{w|Influenza}} || Discovery || Martinon et al. show that subcutaneous injection of liposome-encapsidated mRNA encoding the influenza virus nucleoprotein induces anti-influenza cytotoxic [[w:T cell|T lymphocytes]].<ref name="Rhoads"/> || |
|- | |- | ||
| − | | 1994 || || || Kozak proposes a hypothesis regarding the structural characteristics of the 5' untranslated region (UTR) of mRNA molecules. According to his postulation, these structures could function as regulatory sequences that facilitate translation by enabling proteins to bind and induce conformational changes downstream. This concept suggest that the specific arrangement of nucleotides in the 5' UTR could play a role in modulating the efficiency of protein synthesis. Kozak's hypothesis provide valuable insights into the mechanisms underlying translational control and the potential regulatory functions of mRNA structures.<ref name="Control of ma">{{cite journal |last1=Babendure |first1=Jeremy R. |last2=Babendure |first2=Jennie L. |last3=Ding |first3=Jian-Hua |last4=Tsien |first4=Roger Y. |title=Control of mammalian translation by mRNA structure near caps |journal=RNA|volume=12 |issue=5 |pages=851–861 |doi=10.1261/rna.2309906 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1440912/ |issn=1355-8382}}</ref> || | + | | 1994 || || Hypothesis || Kozak proposes a hypothesis regarding the structural characteristics of the 5' untranslated region (UTR) of mRNA molecules. According to his postulation, these structures could function as regulatory sequences that facilitate translation by enabling proteins to bind and induce conformational changes downstream. This concept suggest that the specific arrangement of nucleotides in the 5' UTR could play a role in modulating the efficiency of protein synthesis. Kozak's hypothesis provide valuable insights into the mechanisms underlying translational control and the potential regulatory functions of mRNA structures.<ref name="Control of ma">{{cite journal |last1=Babendure |first1=Jeremy R. |last2=Babendure |first2=Jennie L. |last3=Ding |first3=Jian-Hua |last4=Tsien |first4=Roger Y. |title=Control of mammalian translation by mRNA structure near caps |journal=RNA|volume=12 |issue=5 |pages=851–861 |doi=10.1261/rna.2309906 |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1440912/ |issn=1355-8382}}</ref> || |
|- | |- | ||
| − | | 1995 || || || Conry et al. publish study on development of mRNA transcripts encoding luciferase and human carcinoembryonic antigen (CEA) as a vaccine vector. The modified mRNA constructs successfully expresses CEA in mouse fibroblasts in vitro and luciferase in vivo after injection. The researchers immunized mice with the mRNA transcripts encoding CEA, resulting in an immune response with anti-CEA antibodies detected in five out of seven immunized mice. This approach shows promise for inducing immune responses against potential cancer-related proteins.<ref name="The DNA Unive"/><ref>{{cite journal |last1=Conry |first1=R. M. |last2=LoBuglio |first2=A. F. |last3=Wright |first3=M. |last4=Sumerel |first4=L. |last5=Pike |first5=M. J. |last6=Johanning |first6=F. |last7=Benjamin |first7=R. |last8=Lu |first8=D. |last9=Curiel |first9=D. T. |title=Characterization of a messenger RNA polynucleotide vaccine vector |journal=Cancer Research |date=1 April 1995 |volume=55 |issue=7 |pages=1397–1400 |url=https://pubmed.ncbi.nlm.nih.gov/7882341/ |issn=0008-5472}}</ref> | + | | 1995 || || Publication || Conry et al. publish study on development of mRNA transcripts encoding luciferase and human carcinoembryonic antigen (CEA) as a vaccine vector. The modified mRNA constructs successfully expresses CEA in mouse fibroblasts in vitro and luciferase in vivo after injection. The researchers immunized mice with the mRNA transcripts encoding CEA, resulting in an immune response with anti-CEA antibodies detected in five out of seven immunized mice. This approach shows promise for inducing immune responses against potential cancer-related proteins.<ref name="The DNA Unive"/><ref>{{cite journal |last1=Conry |first1=R. M. |last2=LoBuglio |first2=A. F. |last3=Wright |first3=M. |last4=Sumerel |first4=L. |last5=Pike |first5=M. J. |last6=Johanning |first6=F. |last7=Benjamin |first7=R. |last8=Lu |first8=D. |last9=Curiel |first9=D. T. |title=Characterization of a messenger RNA polynucleotide vaccine vector |journal=Cancer Research |date=1 April 1995 |volume=55 |issue=7 |pages=1397–1400 |url=https://pubmed.ncbi.nlm.nih.gov/7882341/ |issn=0008-5472}}</ref> |
|- | |- | ||
| − | | 1995 || || || Ian Maclachlan and Pieter Cullis publish a study on the development of a lipid nanoparticle system for the delivery of drugs and gene therapy. This research, conducted at Protiva Biotherapeutics and Inex Pharmaceuticals, introduces a novel approach that utilized lipid nanoparticles to effectively deliver therapeutic agents and genetic material. The study opens up new possibilities in the field of drug delivery and gene therapy by providing a promising platform for targeted and efficient delivery of treatments.<ref>{{cite journal |last1=MacLachlan |first1=Ian |last2=Cullis |first2=Pieter |title=“Diffusible‐PEG‐Lipid Stabilized Plasmid Lipid Particles” |journal=Advances in Genetics |date=2005 |volume=53 |pages=157–188 |doi=10.1016/S0065-2660(05)53006-2}}</ref><ref name="The DNA Unive"/> || | + | | 1995 || || Publication || Ian Maclachlan and Pieter Cullis publish a study on the development of a lipid nanoparticle system for the delivery of drugs and gene therapy. This research, conducted at Protiva Biotherapeutics and Inex Pharmaceuticals, introduces a novel approach that utilized lipid nanoparticles to effectively deliver therapeutic agents and genetic material. The study opens up new possibilities in the field of drug delivery and gene therapy by providing a promising platform for targeted and efficient delivery of treatments.<ref>{{cite journal |last1=MacLachlan |first1=Ian |last2=Cullis |first2=Pieter |title=“Diffusible‐PEG‐Lipid Stabilized Plasmid Lipid Particles” |journal=Advances in Genetics |date=2005 |volume=53 |pages=157–188 |doi=10.1016/S0065-2660(05)53006-2}}</ref><ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 1996 || || || Gilboa and colleagues demonstrate the potential to elicit robust immune responses against tumors in mice by utilizing dendritic cells that were modified with mRNA encoding surface receptors found on the tumors.<ref name="WhatisBiote">{{cite web |title=mRNA is central to production of proteins in the body which makes it an ideal tool for therapeutics and vaccines |url=https://www.whatisbiotechnology.org/index.php/science/summary/mrna/mrna-is-central-to-production-of-proteins-in-the-body-which- |website=WhatisBiotechnology.org |access-date=18 March 2022 |language=en}}</ref> || | + | | 1996 || {{w|Cancer}} || Experiment || Gilboa and colleagues demonstrate the potential to elicit robust immune responses against tumors in mice by utilizing dendritic cells that were modified with mRNA encoding surface receptors found on the tumors.<ref name="WhatisBiote">{{cite web |title=mRNA is central to production of proteins in the body which makes it an ideal tool for therapeutics and vaccines |url=https://www.whatisbiotechnology.org/index.php/science/summary/mrna/mrna-is-central-to-production-of-proteins-in-the-body-which- |website=WhatisBiotechnology.org |access-date=18 March 2022 |language=en}}</ref> || |
|- | |- | ||
| − | | 1996 || | + | | 1996 (August ) || {{w|Cancer}} || Experiment || Boczkowski et al. at {{w|Duke University}} show that dendritic cells modified with mRNA elicit strong immune response against tumours in mice. This experiment shows that mRNA can be used to engineer dendritic cells—key players in the immune system—to produce tumor-specific antigens. When these modified cells are introduced into mice, they trigger an effective immune response targeting the tumors. This groundbreaking work provides early evidence of mRNA’s potential in cancer immunotherapy, paving the way for further development of mRNA-based approaches to enhance the immune system's ability to fight cancer.<ref name="WhatisBiote"/> || {{w|United States}} |
|- | |- | ||
| − | | 1996 || || || | + | | 1996 || || Discovery || RNA interference (RNAi) is discovered, revealing a mechanism in which small RNA molecules regulate gene expression. This groundbreaking discovery would revolutionize molecular biology and earn the Nobel Prize in Physiology or Medicine in 2006 for its discoverers {{w|Andrew Fire}} and {{w|Craig Mello}}. RNAi would since become a crucial tool in scientific research, enabling precise control over gene expression and offering promising applications in therapeutics, agriculture, and biotechnology. || |
|- | |- | ||
| − | | 1997 || || || Canadian American biologist {{w|Jack W. Szostak}} and Richard W. Roberts show that fusions between a synthetic mRNA and its encoded ''myc'' epitope could be enriched from a pool of random sequence mRNA-polypeptide fusions by immunoprecipitation.<ref name="pnas.org">{{cite journal |vauthors=Roberts RW, Szostak JW |date=1997 |title=RNA-peptide fusions for the in vitro selection of peptides and proteins |journal={{w|Proc Natl Acad Sci USA}} |volume=94 |issue=23 |pages=12297–302 |doi=10.1073/pnas.94.23.12297 |pmc=24913 |pmid=9356443|doi-access=free }}</ref> || | + | | 1997 || || Discovery || Canadian American biologist {{w|Jack W. Szostak}} and Richard W. Roberts show that fusions between a synthetic mRNA and its encoded ''myc'' epitope could be enriched from a pool of random sequence mRNA-polypeptide fusions by immunoprecipitation.<ref name="pnas.org">{{cite journal |vauthors=Roberts RW, Szostak JW |date=1997 |title=RNA-peptide fusions for the in vitro selection of peptides and proteins |journal={{w|Proc Natl Acad Sci USA}} |volume=94 |issue=23 |pages=12297–302 |doi=10.1073/pnas.94.23.12297 |pmc=24913 |pmid=9356443|doi-access=free }}</ref> || |
|- | |- | ||
| − | | 1997 || || || Merix Bioscience becomes the first-ever mRNA company, marking a significant milestone in the field of mRNA therapeutics. | + | | 1997 || || Organization || Merix Bioscience, founded as a Duke University spin-out, is established to develop mRNA for cancer vaccines. It becomes the first-ever mRNA company, marking a significant milestone in the field of mRNA therapeutics. The company later changes its name to Argos Therapeutics in 2004.<ref name="The DNA Unive"/><ref name="topic.echemi.co"/><ref>{{cite web |title=Merix Bioscience, Inc |url=https://www.thecasecentre.org/products/view?id=14738 |website=The Case Centre |access-date=9 June 2022 |language=en}}</ref><ref name="WhatisBiote"/> || {{w|United States}} |
|- | |- | ||
| − | | 1997 || || || | + | | 1997 (October) || || Publication || Joel D. Richter publishes the book ''mRNA Formation and Function''. The text delves into the detailed processes of mRNA synthesis, processing, and its crucial role in gene expression. Covering topics such as transcription, splicing, and translation, this work provides comprehensive insights into mRNA's function within cells. The book is a significant resource for understanding the complexities of mRNA biology and its applications in molecular genetics and biotechnology.<ref>{{cite book |last1=Richter |first1=Joel D. |last2=Richter |first2=Professor in the Department of Molecular Genetics and Microbiology Joel D. |title=MRNA Formation and Function |date=16 October 1997 |publisher=Elsevier Science |isbn=978-0-12-587545-5 |url=https://books.google.com.ar/books/about/MRNA_Formation_and_Function.html?id=e_FqAAAAMAAJ&source=kp_book_description&redir_esc=y |language=en}}</ref> || |
|- | |- | ||
| − | | | + | | 1998 || || Discovery || A small interfering RNA (siRNA) or micro RNA (miRNA), is discovered as a class of naturally occurring RNA. This discovery, made by MK and Kostas, reveals that siRNA and miRNA play a crucial role in the post-transcriptional machinery of cells. By targeting specific mRNAs, siRNA and miRNA can form double-stranded RNA molecules that prompt rapid degradation (known as gene silencing). Consequently, this mechanism offers the potential to regulate gene expression at a post-transcriptional level. The utilization of siRNA and miRNA holds promise in the development of treatments for HIV, cancer, and melanoma.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2000 (January) || || Experiment || A significant advancement is made in the field of vaccination when Hoerr, Obst, Ramemenseee, and Jung from the University of Tübingen demonstrate the efficacy of freshly synthesized naked RNA and protamine-protected RNA as viable tools for vaccination. This finding indicates that these RNA molecules could serve as effective agents in vaccine development.<ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | 2000 || | + | | 2000 || || Organization || CureVac, a spin-out company, is established with the purpose of developing mRNA for vaccines. Ingmar Hoerr, the founder of CureVac, plays a crucial role in this endeavor. CureVac AG emerges as the pioneer company to successfully apply mRNA in the field of medicine, marking a significant milestone in the utilization of mRNA technology for medical purposes.<ref name="WhatisBiote"/><ref name="topic.echemi.co">{{cite web |title=Heavy! Global mRNA Pharmaceutical Panorama. |url=https://topic.echemi.com/a/heavy-global-mrna-pharmaceutical-panorama_94571.html |website=topic.echemi.com |access-date=21 April 2022}}</ref> || {{w|Germany}} |
|- | |- | ||
| − | | 2000 || || || | + | | 2000 (October) || || Discovery || A major discovery is made at the University of Pennsylvania by Drew Weissman, H. Ni, D. Scales, Dude, Capodici, McGibney, Abdool, SN Isaacs, Cannon, and Kariko. They report that mRNA encoding for HIV is able to activate a powerful immune response in T cells. This finding opens up new possibilities for the development of mRNA-based vaccines targeting HIV, with the potential to stimulate a robust immune defense against the virus.<ref name="WhatisBiote"/> || {{w|United States}} ({{w|University of Pennsylvania}}) |
|- | |- | ||
| − | | | + | | 2004 (March) || || Discovery || A report indicates that mRNA can activate a series of {{w|Toll-like receptor}}s, which are signaling receptors of the innate immune system. This research was conducted by Kariko, Houping Ni, Capodici, Lamphier, and Drew Weissman from the University of Pennsylvania.<ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | | + | | 2005 (August) || || Discovery || Research team led by {{w|Katalin Karikó}} at {{w|University of Pennsylvania}} finds that {{w|RNA}} is rendered invisible to immune system by replacing its nucleoside {{w|uridine}} with {{w|pseudouridine}}. This modification prevents the immune system from recognizing and attacking the RNA, which is a significant obstacle in the development of RNA-based therapies. Karikó's work is pivotal in overcoming this challenge, paving the way for the development of mRNA vaccines and therapeutics. This discovery would have far-reaching implications in medical research and biotechnology.<ref>{{cite journal |last1=Karikó |first1=Katalin |last2=Buckstein |first2=Michael |last3=Ni |first3=Houping |last4=Weissman |first4=Drew |title=Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA |journal=Immunity |date=August 2005 |volume=23 |issue=2 |pages=165–175 |doi=10.1016/j.immuni.2005.06.008}}</ref><ref name="WhatisBiote"/> || {{w|United States}} ({{w|University of Pennsylvania}}) |
|- | |- | ||
| − | | 2005 || | + | | 2005 (October 19) || || Therapeutic development || MacLachlan and Cullis from Protiva Biotherapeutics and Inex Pharmaceuticals publish an important development in drug delivery and gene therapy. They introduce a lipid nanoparticle system designed for the efficient delivery of drugs and gene therapies. This innovative approach utilizes lipid-based nanoparticles to encapsulate and transport therapeutic agents, offering improved targeting and delivery capabilities.<ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | | + | | 2006 || || Organization || A spin-out company called RNARx is established with the aim of commercializing modified mRNA for the treatment of anaemia. This venture is founded by Kariko and Drew Weissman, who played a crucial role in the development and modification of mRNA technology. The company's focus is to leverage the potential of modified mRNA to address anaemia and explore its therapeutic applications in this specific area. The establishment of RNARx marks a significant step towards translating mRNA research into real-world medical solutions for anaemia treatment.<ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | | + | | 2006 (March 26) || || Experiment || A significant breakthrough is achieved in the field of gene therapy through monkey studies. Researchers conduct experiments using a lipid nanoparticle system to successfully deliver RNA and silence disease-causing genes. This achievement demonstrates the potential of this novel approach for gene therapy. The successful delivery of RNA using the lipid nanoparticle system in monkey studies would pave the way for future advancements in the field of RNA-based therapeutics. The research is conducted under the auspices of Protiva Biotherapeutics, a company at the forefront of developing innovative solutions in the field of gene therapy.<ref name="WhatisBiote"/> || |
|- | |- | ||
| − | | 2006 || || || A | + | | 2006 (December 15) || || Technological advancement || A method for producing mRNA with enhanced stability and translational efficiency is published. The method proposes innovative techniques to improve the characteristics of mRNA, making it more stable and efficient in protein translation. This advancement would opened up new possibilities for mRNA-based applications and further contribute to the development of RNA-based therapies and biotechnological tools.<ref name="WhatisBiote"/> || {{w|Germany}} ({{w|Johannes Gutenberg University}}) |
|- | |- | ||
| − | | | + | | 2008 || || Hypothesis || The initial concept of delivering self-amplifying RepRNA vaccines using synthetic, biodegradable particles is introduced. Since then, the approach of complexing the RNA for targeted delivery to dendritic cells (DCs) would demonstrate its potential through various methods such as polysaccharide, polyplex, and lipoplex formulations.<ref name="Krampsvd">{{cite book |last1=Kramps |first1=Thomas |last2=Elbers |first2=Knut |title=RNA Vaccines: Methods and Protocols |date=2017 |publisher=Springer New York |isbn=978-1-4939-6481-9 |url=https://books.google.com.ar/books/about/RNA_Vaccines.html?id=NN-EAQAACAAJ&source=kp_book_description&redir_esc=y |language=en}}</ref><ref>{{cite web |last1=Tratschin |first1=Jon Duri |last2=Ruggli |first2=Nicolas |last3=McCullough |first3=Kenneth Charles |title=Pestivirus replicons providing an rna-based viral vector system |url=https://patents.google.com/patent/WO2009146867A1/en |access-date=5 March 2022 |date=10 December 2009}}</ref> || |
|- | |- | ||
| − | | | + | | 2008 || || Organization || Biopharmaceutical New Technologies ({{w|BioNTech}}) is established with the aim of utilizing mRNA for the development of personalized cancer immunotherapies. The founders recognize the potential of mRNA technology in the field of cancer treatment and seek to harness its capabilities for more accurate and individualized immunotherapy approaches. The establishment of BioNTech marks a significant event in the advancement of mRNA-based therapies, with a focus on tailoring treatments to the specific needs of cancer patients. Since its inception, BioNTech would be dedicated to pushing the boundaries of immunotherapy and would play a role in the development of innovative mRNA-based cancer treatments.<ref name="WhatisBiote"/><ref name="topic.echemi.co"/> || {{w|Germany}} |
|- | |- | ||
| − | | | + | | 2009 || Metastatic melanoma || Clinical trial || A groundbreaking trial is conducted on mRNA-based vaccines for cancer immunotherapy in individuals with metastatic melanoma. The trial demonstrates the potential of mRNA technology to stimulate the immune system and generate vaccine-specific T cells targeted against melanoma. The results show a significant increase in the number of T cells directed against melanoma, indicating the vaccines' ability to elicit an immune response. This trial marks a significant advancement in the development of mRNA-based vaccines for cancer treatment and provides promising evidence for their use in cancer {{w|immunotherapy}}.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2010 || || Technological advancement || Derrick Rossi, a researcher at Harvard Medical School, expands upon Shinya Yamanaka's breakthrough in inducing pluripotency and utilizes RNA to reprogram adult cells into embryonic stem cells. This significant development paves the way for the establishment of Moderna Therapeutics, as it harnesses the potential of RNA technology for various applications in medicine and therapeutics.<ref name="The DNA Unive"/><ref>{{cite journal |last1=Warren |first1=Luigi |last2=Manos |first2=Philip D. |last3=Ahfeldt |first3=Tim |last4=Loh |first4=Yuin-Han |last5=Li |first5=Hu |last6=Lau |first6=Frank |last7=Ebina |first7=Wataru |last8=Mandal |first8=Pankaj K. |last9=Smith |first9=Zachary D. |last10=Meissner |first10=Alexander |last11=Daley |first11=George Q. |last12=Brack |first12=Andrew S. |last13=Collins |first13=James J. |last14=Cowan |first14=Chad |last15=Schlaeger |first15=Thorsten M. |last16=Rossi |first16=Derrick J. |title=Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA |journal=Cell Stem Cell |date=5 November 2010 |volume=7 |issue=5 |pages=618–630 |doi=10.1016/j.stem.2010.08.012 |url=https://pubmed.ncbi.nlm.nih.gov/20888316/ |issn=1875-9777}}</ref> || {{w|United States}} ({{w|Harvard Medical School}}) |
|- | |- | ||
| − | | | + | | 2010 || || Technological advancement || Eduard Yakubov et al. develop a method to reprogram human fibroblasts into induced pluripotent cells (iPS) using mRNA derived from specific transcription factors. Their approach aim to reduce the risks associated with DNA integration by traditional reprogramming methods. By transfecting the cells with RNA synthesized from cDNA of the transcription factors, the researchers observed successful intracellular protein expression and nuclear localization. Through multiple transfections, iPS colonies expressing embryonic stem cell markers were generated. Importantly, this RNA transfection approach eliminates the need for DNA integration and holds promise as a potential alternative to DNA vectors in iPS generation..<ref>{{cite journal |last1=Yakubov |first1=Eduard |last2=Rechavi |first2=Gidi |last3=Rozenblatt |first3=Shmuel |last4=Givol |first4=David |title=Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors |journal=Biochemical and Biophysical Research Communications |date=26 March 2010 |volume=394 |issue=1 |pages=189–193 |doi=10.1016/j.bbrc.2010.02.150 |url=https://pubmed.ncbi.nlm.nih.gov/20188704/ |issn=1090-2104}}</ref><ref name="The DNA Unive"/> || {{w|Israel}} ({{w|Weizmann Institute of Science}}) |
|- | |- | ||
| − | | 2010 || || || | + | | 2010 (November) || || Organization || Moderna Therapeutics is established with the objective of commercializing modified mRNA vaccines and therapeutics. Founded by Rossi, Kernneth Chien, and Robert Langer, Moderna enters the scene as the final member of the "mRNA Big Three" companies to be established. Despite being the last to emerge, Moderna would quickly gain significant recognition and is highly valued, surpassing the other two companies in the field. With a focus on mRNA therapies, Moderna would make notable progress in the development of innovative treatments using mRNA as a therapeutic agent.<ref name="topic.echemi.co"/><ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 2010 || || || | + | | 2010 (November 5) || || Technological advancement || A team led by Luigi Warren, Philip Manos, and Chad Cowan report the successful transformation of skin cells into pluripotent stem cells using modified mRNA. The researchers utilized modified mRNA to reprogram the skin cells into a pluripotent state, capable of differentiating into various cell types.<ref name="The DNA Unive"/> || {{w|United States}} ({{w|Harvard University}}) |
|- | |- | ||
| − | | | + | | 2011 || || Partnership || CureVac establishes a partnership with Sanofi Pasteur, the vaccines division of Sanofi. This collaboration focuses on developing mRNA-based vaccines, combining CureVac’s expertise in mRNA technology with Sanofi Pasteur's experience in vaccine development. The partnership aims to explore new approaches to vaccine design and production, leveraging mRNA’s potential to create more effective and flexible vaccines for various infectious diseases. This alliance is part of a broader trend in the pharmaceutical industry to invest in innovative technologies for advancing public health. |
|- | |- | ||
| − | | | + | | 2012 || || Discovery || Breakthroughs in genetic engineering are made by two separate teams led by {{w|Jennifer Doudna}} and {{w|Emmanuelle Charpentier}}. Their work focuses on utilizing {{w|CRISPR}} for precise genome editing. The CRISPR system, consisting of the {{w|Cas9}} enzyme and a guide {{w|RNA}}, is found to target specific sequences in the genome. Plasmids or viral vectors are used to introduce these components into target cells. However, the sustained presence of Cas9-encoding plasmid DNA can lead to unintended modifications in non-targeted regions of the genome. These discoveries revolutionize genetic engineering and offer new opportunities for precise {{w|genome editing}}.<ref>{{cite journal |last1=Jinek |first1=Martin |last2=Chylinski |first2=Krzysztof |last3=Fonfara |first3=Ines |last4=Hauer |first4=Michael |last5=Doudna |first5=Jennifer A. |last6=Charpentier |first6=Emmanuelle |title=A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity |journal=Science (New York, N.Y.) |date=17 August 2012 |volume=337 |issue=6096 |pages=816–821 |doi=10.1126/science.1225829 |url=https://pubmed.ncbi.nlm.nih.gov/22745249/ |issn=1095-9203}}</ref><ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2013 || Cancer || Organization|| RNA Immunotherapies, a Belgian biotechnology company, is founded with the objective of developing immunotherapy for cancer and infectious diseases. The company is established in collaboration with Professor Kris Thielemans, a renowned immunologist from the Free University of Brussels, along with other partners. Their focus is on utilizing their proprietary mRNA TriMix platform for the development of innovative immunotherapies.<ref name="topic.echemi.co"/> || {{w|Belgium}} |
|- | |- | ||
| − | | | + | | 2013 || Cardiometabolic diseases, cancer || Partnership || {{w|Moderna}} partners with {{w|AstraZeneca}} to develop messenger RNA therapeutics for cardiometabolic diseases and cancer. This collaboration, involving a $240 million upfront payment from AstraZeneca, focuses on a novel treatment approach that enables the body to produce therapeutic proteins in vivo. AstraZeneca gains exclusive rights to develop up to 40 drug products over five years, with Moderna entitled to up to $180 million in milestone payments and royalties. Moderna's technology aims to create innovative drugs for previously undruggable targets, with AstraZeneca leading the clinical development and commercialization efforts.<ref name="topic.echemi.co"/><ref>{{cite web |title=AstraZeneca and Moderna Therapeutics Announce Collaboration for Cardiometabolic Diseases and Cancer Treatment |url=https://www.astrazeneca.com/media-centre/press-releases/2013/astrazeneca-moderna-therapeutics-cardiometabolic-diseases-cancer-treatment-21032013.html# |website=AstraZeneca |date=21 March 2013 |accessdate=16 September 2024}}</ref> || {{w|United States}}, {{w|United Kingdom}}, {{w|Sweden}} |
|- | |- | ||
| − | | 2013 || || || {{w|CureVac}} partners with {{w|Johnson and Johnson}}'s {{w|Janssen Pharmaceuticals}} to develop a mRNA flu vaccine.<ref name="topic.echemi.co"/> || | + | | 2013 || {{w|Influenza}} || Partnership || {{w|CureVac}} partners with {{w|Johnson and Johnson}}'s {{w|Janssen Pharmaceuticals}} to develop a mRNA flu vaccine. This collaboration aims to leverage CureVac's expertise in mRNA technology to create a novel vaccine platform for influenza. The partnership seeks to advance the development of an mRNA vaccine that can potentially offer more effective and adaptable protection against flu viruses compared to traditional vaccine approaches. This initiative underscored the growing interest and investment in mRNA technology as a versatile tool for combating infectious diseases.<ref name="topic.echemi.co"/> || |
|- | |- | ||
| − | | 2013 || || || | + | | 2013 (October) || Cardiovascular diseases || Experiment || A group of researchers from Harvard University, Massachusetts General Hospital, Children's Hospital Boston, Mount Sinai School of Medicine, and Karolinska Institute, conduct a study that demonstrates the potential of modified mRNA in improving heart function in mice. Through their research, they utilized modified mRNA and observed significant improvements in heart function in the mice subjects. The findings of this study highlight the therapeutic efficacy of modified mRNA in enhancing heart function. This discovery would open up promising avenues for further investigations into the use of modified mRNA as a potential treatment for heart-related conditions. The study provides valuable insights and set the stage for future research in the development of novel therapies for cardiovascular diseases.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2014 || Infectious diseases, Hypersensitivities, Cancer || Clinical trial || Numerous pre-clinical and clinical trials start being carried out to investigate the utilization of mRNA technology in the development of vaccines targeting infectious diseases, hypersensitivities, and cancer. These trials, as documented by Sahin et al. (2014) and Weissman (2015), encompass a range of studies that aim to evaluate the effectiveness and safety of mRNA-based vaccines in combating these conditions.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2015 (May 11) || Cancer || Partnership || BioNTech announces a partnership agreement with Eli Lilly, with the purpose to advance the development of new tumor immunotherapy treatments, specifically focusing on T-cell receptor (TCR) therapies. The partnership involved a significant financial commitment, with Lilly investing more than $360 million in the collaboration. The objective of the collaboration was to leverage BioNTech's expertise in mRNA technology and Lilly's experience in the development and commercialization of innovative therapies. By combining their resources and knowledge, the two companies aim to accelerate the progress of tumor immunotherapy treatments, with a particular emphasis on TCR therapies.<ref>{{cite web |title=Lilly and BioNTech Announce Research Collaboration on Novel Cancer Immunotherapies |url=https://investor.lilly.com/news-releases/news-release-details/lilly-and-biontech-announce-research-collaboration-novel-cancer |website=investor.lilly.com |access-date=4 June 2023}}</ref><ref name="topic.echemi.co"/> || |
|- | |- | ||
| − | | | + | | 2015 || Immunity || Discovery || {{w|Katalin Karikó}} and her colleague Drew Weissmann discover a solution to prevent the activation of the immune response triggered by injected mRNA. It is observed that mRNA activates {{w|toll-like receptor}}s (TLR) present on immune cells. To address this, Karikó and Weissmann introduced a modification to the RNA by incorporating a naturally occurring modified nucleoside known as {{w|pseudouridine}}.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2016 || Animal health, Cancer || Partnership || BioNTech establishes collaborations with two major companies. The first is with Bayer, aiming to develop innovative mRNA vaccines and drugs for animal health. This partnership aims to leverage BioNTech's mRNA technology platform to advance veterinary healthcare. In September of the same year, BioNTech joins forces with Genentech, a subsidiary of Roche, to develop an individualized mRNA tumor vaccine. This vaccine is designed to target specific neoantigens, which are unique to each patient's tumor. By utilizing mRNA technology, the goal is to develop personalized cancer immunotherapy that could effectively target and treat tumors based on the patient's specific genetic makeup.<ref name="topic.echemi.co"/> || |
|- | |- | ||
| − | | | + | | 2016 || || Technological advancement || The development of CRISPR-Cas9 technology revolutionizes the field of {{w|genetic engineering}} by providing a precise and versatile tool for gene editing. This technology uses a bacterial immune system to make targeted cuts in DNA, allowing scientists to modify specific genes with high accuracy. The advent of CRISPR-Cas9 opens new possibilities for manipulating mRNA by enabling the correction of genetic mutations that cause diseases, optimizing mRNA expression, and studying gene function. Its potential applications extend to treating genetic disorders, advancing research in functional genomics, and developing innovative therapies, including those that leverage mRNA technology.<ref>{{cite journal |title=CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing |journal=PLOS ONE |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9377665/ |accessdate=16 September 2024 |doi=10.1371/journal.pone.0273864}}</ref> |
|- | |- | ||
| − | | 2016 || || || | + | | 2016 (November) || Genetic disorders || Organization || Translate Bio, a Massachusetts-based company specializing in RNA technology, is officially registered under its original name, RaNA Therapeutics. The company's focus is on developing mRNA-based therapeutics for various diseases, leveraging the potential of RNA to address genetic disorders and other conditions. The rebranding from RaNA Therapeutics to Translate Bio reflects its commitment to advancing RNA science and its applications in medicine. This change marks a significant step in the company's evolution and its continued efforts to harness mRNA technology for innovative therapeutic solutions.<ref name="topic.echemi.co"/> || {{w|United States}} |
|- | |- | ||
| − | | | + | | 2017–2019 || HIV, Rabies || Scientific advancement || Significant advancements are achieved in the effectiveness of mRNA encoding antibodies against HIV and rabies, as evidenced by studies conducted by Pardi et al., Thran et al., and Schlake et al. in 2019. These studies contribute to the growing understanding of the potential of mRNA-based approaches in combating these infectious diseases.<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2017 || Cancer || Experiment || In a study conducted by Stadler et al., researchers demonstrate the effectiveness of mRNA-encoded bispecific antibodies in eliminating large tumors in mice. By using in vitro-transcribed, pharmacologically optimized mRNA encoding the antibody, they achieved sustained endogenous synthesis of the antibody in the mice. The results show that the mRNA-encoded antibodies are as effective as purified bispecific antibodies in eliminating advanced tumors. This approach offers a potential solution to manufacturing challenges and could expedite the clinical development of novel bispecific antibodies due to the fast production of pharmaceutical mRNA.<ref name="The DNA Unive"/><ref>{{cite journal |last1=Stadler |first1=Christiane R. |last2=Bähr-Mahmud |first2=Hayat |last3=Celik |first3=Leyla |last4=Hebich |first4=Bernhard |last5=Roth |first5=Alexandra S. |last6=Roth |first6=René P. |last7=Karikó |first7=Katalin |last8=Türeci |first8=Özlem |last9=Sahin |first9=Ugur |title=Elimination of large tumors in mice by mRNA-encoded bispecific antibodies |journal=Nature Medicine |date=July 2017 |volume=23 |issue=7 |pages=815–817 |doi=10.1038/nm.4356 |url=https://www.nature.com/articles/nm.4356 |language=en |issn=1546-170X}}</ref> || |
|- | |- | ||
| − | | | + | | 2017 (October) || Cancer || Partnership || {{w|CureVac}} enters into a strategic partnership with {{w|Eli Lilly}} to jointly develop and commercialize five cancer vaccine products. This collaboration aims to leverage CureVac's expertise in mRNA-based therapies for cancer treatment. Additionally, in November of the same year, CureVac forms a partnership with CRISPR to advance the development of Cas9 mRNA for in vivo gene editing. These partnerships highlight the direction aimed at advancing innovative mRNA technologies for therapeutic applications in cancer treatment and gene editing.<ref name="topic.echemi.co"/> || {{w|United States}} |
|- | |- | ||
| − | | | + | | 2018 || || Partnership || CureVac and Arcturus form a strategic partnership aimed at collaborating on the identification, development, and commercialization of novel mRNA therapies. This partnership brings together the expertise and resources of both companies to advance the field of mRNA-based therapeutics and explore new treatment possibilities.<ref name="topic.echemi.co"/> || |
|- | |- | ||
| − | | | + | | 2019 || {{w|Cancer}} || Partnership|| Danish biotechnology company {{w|Genmab}} and {{w|CureVac}} partner to develop mRNA-based antibody therapeutics. The collaboration aims to combine CureVac's mRNA technology with Genmab's antibody expertise to create innovative treatment options for cancer patients. Genmab agrees to provide CureVac with a $10 million upfront payment and make a €20 million equity investment. The companies agree to conduct joint research to identify an initial product candidate, with Genmab taking responsibility for its development and commercialization. The partnership is expected to contribute to advancing mRNA-based antibody therapeutics in the field of cancer treatment.<ref name="topic.echemi.co"/><ref>{{cite web |title=Genmab and CureVac Enter Strategic Partnership to Develop mRNA-based Antibody Therapeutics |url=https://ir.genmab.com/news-releases/news-release-details/genmab-and-curevac-enter-strategic-partnership-develop-mrna |website=ir.genmab.com |access-date=5 June 2023}}</ref> || |
|- | |- | ||
| − | | | + | | 2019 || {{w|HIV}} || Experiment || A study conducted by Pardi et al. demonstrates the efficacy of nucleoside-modified mRNA in encoding a broadly neutralizing antibody against HIV-1. The researchers generated nucleoside-modified mRNAs encoding the light and heavy chains of the anti-HIV-1 antibody VRC01 and administered them to mice using lipid nanoparticles. The systemic administration of the mRNA resulted in significant levels of the VRC01 antibody in the plasma. Weekly injections of the mRNA maintained sustained antibody levels in mice. Importantly, a single injection of VRC01 mRNA provided protection against intravenous HIV-1 challenge in humanized mice. This study highlights the potential of nucleoside-modified mRNA as a delivery platform for passive immunotherapy against HIV-1 and potentially other diseases.<ref name="The DNA Unive"/><ref>{{cite journal |last1=Pardi |first1=Norbert |last2=Secreto |first2=Anthony J. |last3=Shan |first3=Xiaochuan |last4=Debonera |first4=Fotini |last5=Glover |first5=Joshua |last6=Yi |first6=Yanjie |last7=Muramatsu |first7=Hiromi |last8=Ni |first8=Houping |last9=Mui |first9=Barbara L. |last10=Tam |first10=Ying K. |last11=Shaheen |first11=Farida |last12=Collman |first12=Ronald G. |last13=Karikó |first13=Katalin |last14=Danet-Desnoyers |first14=Gwenn A. |last15=Madden |first15=Thomas D. |last16=Hope |first16=Michael J. |last17=Weissman |first17=Drew |title=Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge |journal=Nature Communications |date=2 March 2017 |volume=8 |pages=14630 |doi=10.1038/ncomms14630 |url=https://pubmed.ncbi.nlm.nih.gov/28251988/ |issn=2041-1723}}</ref> || |
|- | |- | ||
| − | | | + | | 2019 || {{w|Toxin}}s || Therapeutic development || It is reported that mRNA technology could be utilized for the production and administration of antibodies against toxins. In cases where individuals are exposed to venom or toxins, immediate neutralization is crucial. Normally, it takes the body several days or weeks to generate antibodies, but a rapid response is essential in such situations. Traditionally, antibodies against toxins are produced using expensive and less accessible mammalian cell cultures. However, mRNA technology offers a more direct approach for antibody production. These antibodies can be administered through various routes such as intravenous, intradermal, subcutaneous, intramuscular, or intranodal, allowing for a swift response. Therefore, mRNA-mediated neutralization presents a promising and fast-acting alternative to conventional antibody use.<ref>{{cite journal |last1=Schlake |first1=Thomas |last2=Thran |first2=Moritz |last3=Fiedler |first3=Katja |last4=Heidenreich |first4=Regina |last5=Petsch |first5=Benjamin |last6=Fotin-Mleczek |first6=Mariola |title=mRNA: A Novel Avenue to Antibody Therapy? |journal=Molecular Therapy: The Journal of the American Society of Gene Therapy |date=10 April 2019 |volume=27 |issue=4 |pages=773–784 |doi=10.1016/j.ymthe.2019.03.002 |url=https://pubmed.ncbi.nlm.nih.gov/30885573/ |issn=1525-0024}}</ref><ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | | + | | 2019 || || Experiment || Lindsay et al. conduct a study on mRNA vaccine behavior within the body, focusing on its uptake and expression by immune cells. They observe a strong mRNA signal in the {{w|lymphoid node}} within 4 hours of intramuscular administration. This successful accumulation of mRNA in the lymphoid node highlights the importance of administration routes for vaccine distribution and immune cell uptake. The findings provide valuable insights into mRNA vaccine behavior, emphasizing the role of administration routes in shaping the immune response. Understanding the timing and localization of mRNA uptake in the lymphoid node contributes to optimizing vaccine delivery strategies and enhancing their effectiveness.<ref name="The DNA Unive"/> || {{w|United States}} ({{w|Georgia Tech}}, {{w|Emory University}}) |
|- | |- | ||
| − | | 2019 || || || | + | | 2019 (December 6) || || Publication || Marlene Oeffinger and Daniel Zenklusen publish ''The Biology of mRNA: Structure and Function'', which provides an in-depth exploration of the biology of messenger RNA (mRNA), focusing on its structure, function, and role in cellular processes. It covers the mechanisms of mRNA synthesis, processing, and translation, as well as its regulatory aspects and implications for gene expression. The book is aimed at researchers and students in molecular biology, genetics, and related fields, offering comprehensive insights into the fundamental aspects of mRNA and its impact on cellular and molecular biology.<ref>{{cite book |last1=Oeffinger |first1=Marlene |last2=Zenklusen |first2=Daniel |title=The Biology of mRNA: Structure and Function |date=6 December 2019 |publisher=Springer Nature |isbn=978-3-030-31434-7 |url=https://books.google.com.ar/books/about/The_Biology_of_MRNA_Structure_and_Functi.html?id=9T7CDwAAQBAJ&source=kp_book_description&redir_esc=y |language=en}}</ref> || |
|- | |- | ||
| − | | 2019 || || || | + | | 2019 (December) || {{w|COVID-19}} || Background || The emergence of the {{w|COVID-19 pandemic}} highlights an urgent global need for new vaccines. The novel coronavirus, SARS-CoV-2, quickly spreads across the world, causing widespread illness and significant disruptions. The rapid increase in cases and severity of the disease spurs unprecedented efforts in vaccine research and development. Scientists and pharmaceutical companies worldwide focus on creating effective vaccines to curb the spread of the virus and protect public health. This urgent need for vaccines leads to accelerated research, collaboration, and the eventual development and approval of several COVID-19 vaccines within a remarkably short timeframe. || Worldwide |
|- | |- | ||
| − | | | + | | 2020 || {{w|COVID-19}} || Vaccine development || Pfizer and BioNTech announce their collaboration to co-develop a potential mRNA-based vaccine, called BNT162, for preventing COVID-19 infection. The companies signed a letter of intent and a Material Transfer and Collaboration Agreement to begin working together immediately. The collaboration aims to accelerate the development of the vaccine. This partnership builds on their previous agreement to develop mRNA-based vaccines for influenza.<ref>{{cite web |title=Pfizer and BioNTech to Co-Develop Potential COVID-19 Vaccine {{!}} Pfizer |url=https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-co-develop-potential-covid-19-vaccine |website=www.pfizer.com |access-date=5 June 2023}}</ref><ref name="topic.echemi.co"/> || {{w|United States}}, {{w|Germany}} |
|- | |- | ||
| − | | | + | | 2020 (July) || {{w|Infectious diseases}} || Partnership || {{w|GlaxoSmithKline}} (GSK) and {{w|CureVac}} announce a strategic collaboration agreement for the development of mRNA-based vaccines and monoclonal antibodies (mAbs) targeting infectious diseases. The partnership aims to leverage CureVac's expertise in mRNA technology and GSK's scientific leadership in vaccines and mAbs. The companies agree to work on up to five mRNA-based vaccines and mAbs, with a focus on infectious disease pathogens. GSK agrees to invest £130 million (€150 million) in CureVac, representing a 10% stake, and provide an upfront payment of £104 million (€120 million). CureVac would be responsible for preclinical and clinical development through Phase 1 trials, after which GSK will take over further development and commercialization. The collaboration is expected to contribute to advancing mRNA-based vaccine and treatment technologies and improving responses to future pandemics.<ref name="topic.echemi.co"/><ref>{{cite web |title=GSK and CureVac announce strategic mRNA technology collaboration {{!}} GSK |url=https://www.gsk.com/en-gb/media/press-releases/gsk-and-curevac-announce-strategic-mrna-technology-collaboration/ |website=www.gsk.com |access-date=5 June 2023 |date=20 July 2020}}</ref> || {{w|United Kingdom}}, {{w|Germany}} |
|- | |- | ||
| − | | | + | | 2020 (December) || {{w|COVID-19}} || Vaccine development || [[w:BioNTech|Pfizer–BioNTech]] and {{w|Moderna}} obtain authorization for their mRNA-based COVID-19 vaccines. "In 2020, the FDA approved the first mRNA-based vaccines against an infectious disease SARS-CoV-2. This was only made possible by decades of research on mRNA-based therapeutics."<ref name="The DNA Unive"/> || |
|- | |- | ||
| − | | 2020 || || || | + | | 2020 (December 11) || {{w|COVID-19}} || Vaccine development || The U.S. {{w|FDA}} approves the first mRNA vaccine for COVID-19 for emergency use, by [[w:Pfizer–BioNTech COVID-19 vaccine|BioNTech and Pfizer]]. This landmark approval marks a significant advancement in the pandemic response, introducing a new vaccine technology based on messenger RNA (mRNA). The Pfizer-BioNTech vaccine, branded as Comirnaty, works by instructing cells to produce a protein similar to the spike protein of the SARS-CoV-2 virus, thereby triggering an immune response. This authorization is a pivotal moment in the global effort to control COVID-19 and laid the groundwork for subsequent vaccine approvals.<ref name="WhatisBiote"/><ref>{{cite web |last1=Commissioner |first1=Office of the |title=FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine |url=https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 |website=FDA |access-date=10 June 2022 |language=en |date=14 December 2020}}</ref> || {{w|United States}} |
|- | |- | ||
| − | | 2020 || | + | | 2020 (December 18) || {{w|COVID-19}} || Vaccine development || The U.S. {{w|FDA}} approves [[w:Moderna COVID-19 vaccine|second mRNA vaccine for COVID-19]], by {{w|Moderna}}. This vaccine, known as the Moderna COVID-19 vaccine, follows the earlier approval of the Pfizer-BioNTech vaccine, marking a significant milestone in the fight against the pandemic. The mRNA technology used in these vaccines represents a novel approach, instructing cells to produce a protein that triggers an immune response. This authorization expands the options available for vaccination, helping to accelerate the global vaccination campaign and combat the spread of the virus.<ref name="WhatisBiote"/> || {{w|United States}} |
|- | |- | ||
| − | | | + | | 2021 || {{w|Cancer}} || Therapeutic development || Miao et al. report that mRNA encoding antibodies targeting tumors is an area of active research. Monoclonal antibodies (mAbs) have the ability to directly kill cancer cells or stimulate immune cells to combat cancer. Bispecific antibodies, which can bind to both T cells and cancer cells, are particularly noteworthy as they facilitate the interaction between T cells and cancer cells, leading to the destruction of cancer cells by T cells. At this time, clinical trials currently evaluate mRNA-based vaccines for various types of cancer, including colorectal cancer, prostate cancer, triple-negative breast cancer, bladder cancer, pancreatic cancer, esophageal squamous carcinoma, gastric adenocarcinoma, melanoma, and non-small cell lung carcinoma.<ref name="The DNA Unive"/><ref>{{cite journal |last1=Miao |first1=Zhen |last2=Balzer |first2=Michael S. |last3=Ma |first3=Ziyuan |last4=Liu |first4=Hongbo |last5=Wu |first5=Junnan |last6=Shrestha |first6=Rojesh |last7=Aranyi |first7=Tamas |last8=Kwan |first8=Amy |last9=Kondo |first9=Ayano |last10=Pontoglio |first10=Marco |last11=Kim |first11=Junhyong |last12=Li |first12=Mingyao |last13=Kaestner |first13=Klaus H. |last14=Susztak |first14=Katalin |title=Single cell regulatory landscape of the mouse kidney highlights cellular differentiation programs and disease targets |journal=Nature Communications |date=15 April 2021 |volume=12 |issue=1 |pages=2277 |doi=10.1038/s41467-021-22266-1 |url=https://www.nature.com/articles/s41467-021-22266-1 |language=en |issn=2041-1723}}</ref> || |
|- | |- | ||
| − | | | + | | 2022 (November) || Multiple || Publication || Sarfaraz K. Niazi publishes ''mRNA Therapeutics: Fast-to-Market Strategies'', a 326-page guide covering nucleic acid therapeutics, focusing on mRNA vaccines. The book details manufacturing, formulations, safety testing, and regulatory steps, emphasizing cGMP compliance. It introduces the concept of "biosimilar" mRNA vaccines for rapid approval, providing financial projections for RNA manufacturing facilities and insights into supply chain management. The text also explores the future of nucleic acid products, suggesting a shift towards nucleic acid vaccines for autoimmune disorders' prevention. The book anticipates increased industry engagement, promising significant advancements in drug development.<ref>{{cite web |last1=Niazi |first1=Sarfaraz K. |title=mRNA Therapeutics: Fast-to-Market Strategies |url=https://books.google.com.ar/books/about/MRNA_Therapeutics.html?id=xMeVEAAAQBAJ&source=kp_book_description&redir_esc=y |website=books.google.com.ar |publisher=CRC Press |access-date=3 October 2023 |language=en |date=6 December 2022}}</ref> |
|- | |- | ||
| − | | | + | | 2022 (December 29) || {{w|Melanoma}} || Therapeutic development || A study on {{w|melanoma}} treatment unveils promising results with personalized mRNA vaccines combined with the immunotherapy drug {{w|pembrolizumab}}. In the KEYNOTE/mRNA trial, the combination therapy significantly reduces the risk of recurrence or death by 44% compared to {{w|pembrolizumab}} alone in stage III/IV melanoma patients post-surgery. Although there were slightly more adverse events with the combined therapy, experts hail this advancement as a significant step in melanoma treatment. Personalized mRNA vaccines train the immune system to recognize cancer proteins, potentially revolutionizing cancer therapy. This development, reminiscent of the penicillin moment, indicates a promising future for mRNA vaccines in treating various cancers.<ref>{{cite web |title=Breakthrough in mRNA vaccines for melanoma |url=https://www1.racgp.org.au/newsgp/clinical/breakthrough-in-mrna-vaccines-for-melanoma |website=racgp.org.au |access-date=27 May 2024}}</ref> |
|- | |- | ||
| − | | | + | | 2023 (October 2) || {{w|COVID-19}} || Recognition || The {{w|Nobel Prize in Physiology or Medicine}} is jointly awarded to {{w|Katalin Karikó}} and {{w|Drew Weissman}} for their discoveries in [[w:Nucleoside-modified messenger RNA|nucleoside base modifications]], which paved the way for the development of highly effective mRNA vaccines against {{w|COVID-19}}. Their research revolutionized our understanding of mRNA interaction with the {{w|immune system}}, facilitating the rapid development of vaccines during the pandemic. Before the pandemic, vaccines relied on whole viruses, viral components, or vector systems, requiring large-scale cell culture. Karikó and Weissman's work on mRNA technology overcame previous obstacles, offering a promising avenue for vaccine development independent of cell culture. Their breakthrough, published in 2005, anticipated the mRNA vaccine revolution witnessed during the COVID-19 pandemic.<ref>{{cite web |title=The Nobel Prize in Physiology or Medicine 2023 |url=https://www.nobelprize.org/prizes/medicine/2023/press-release/ |website=NobelPrize.org |access-date=27 May 2024}}</ref> |
|- | |- | ||
| − | | | + | | 2024 (April 7) || {{w|Pancreatic cancer}} || Therapeutic development || In a phase 1 clinical trial, the investigational mRNA-based cancer vaccine autogene cevumeran shows promising results in stimulating a lasting immune response against pancreatic cancer. Administered alongside {{w|atezolizumab}} and {{w|mFOLFIRINOX}} {{w|chemotherapy}}, the vaccine activates {{w|T cell}}s specific to neoantigens found in individual patients' tumors, potentially reducing the risk of cancer recurrence. Notably, vaccine-induced T cells persisted for up to three years after treatment in some patients. These findings support the development of personalized mRNA vaccines as a viable approach to targeting pancreatic cancer and warrant further investigation in larger clinical trials.<ref>{{cite web |last1=Stallard |first1=Jim |title=Investigational mRNA Vaccine Induced Persistent Immune Response in Phase 1 Trial of Patients With Pancreatic Cancer {{!}} Memorial Sloan Kettering Cancer Center |url=https://www.mskcc.org/news/can-mrna-vaccines-fight-pancreatic-cancer-msk-clinical-researchers-are-trying-find-out |website=www.mskcc.org |access-date=28 May 2024 |language=en |date=7 April 2024}}</ref> |
|- | |- | ||
|} | |} | ||
| Line 281: | Line 294: | ||
===What the timeline is still missing=== | ===What the timeline is still missing=== | ||
| − | + | ||
| + | * Summary by decade | ||
| + | * https://www.science.org/content/article/mrna-discovery-paved-way-covid-19-vaccines-wins-nobel-prize-physiology-medicine multiple events | ||
| + | * https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/ multiple events | ||
| + | * https://the-dna-universe.com/2021/04/15/the-history-of-mrna-applications/ ref name="The DNA Unive"> multiple events | ||
| + | * https://www.pfizer.com/science/innovation/mrna-technology by decade | ||
| + | * https://www.theguardian.com/society/2024/apr/26/cancer-mrna-vaccine-melanoma-trial | ||
| + | * https://www.mskcc.org/news/can-mrna-vaccines-fight-pancreatic-cancer-msk-clinical-researchers-are-trying-find-out | ||
| + | * https://www.biospace.com/article/releases/mrna-therapeutics-market-size-set-to-soar-usd-58-90-bn-by-2033/ | ||
* https://theconversation.com/brain-cancer-in-children-is-notoriously-hard-to-treat-a-new-mrna-cancer-vaccine-triggers-an-attack-from-within-228666 | * https://theconversation.com/brain-cancer-in-children-is-notoriously-hard-to-treat-a-new-mrna-cancer-vaccine-triggers-an-attack-from-within-228666 | ||
| − | |||
* [https://www.bristows.com/news/spotlight-on-mrna-a-brief-history-of-mrna-research/] | * [https://www.bristows.com/news/spotlight-on-mrna-a-brief-history-of-mrna-research/] | ||
* [https://www.theatlantic.com/ideas/archive/2021/03/how-mrna-technology-could-change-world/618431/] | * [https://www.theatlantic.com/ideas/archive/2021/03/how-mrna-technology-could-change-world/618431/] | ||
Latest revision as of 20:45, 21 September 2024
This is a timeline of mRNA research. Today, mRNA is a widely recognized and potent tool for generating antigen-specific immunity in the field of vaccines and immunotherapy.[1]
Contents
Sample questions
The following are some interesting questions that can be answered by reading this timeline:
- What are the important discoveries that contributed to the identification and understanding of mRNA?
- Sort the full timeline by "Event type" and look for the group of rows with value "Discovery".
- You will see a chronology of significant scientific discoveries related to mRNA research, highlighting key milestones, influential experiments, and important figures in the field.
- What are some milestone experiments in mRNA research that contributed to modern medicine?
- Sort the full timeline by "Event type" and look for the group of rows with value "Experiment".
- You will receive a chronological list of key experiments related to mRNA research. These experiments highlight significant advancements in understanding mRNA's role in gene expression, protein synthesis, and its therapeutic potential, including applications in cancer immunotherapy, cardiovascular treatments, gene therapy, and vaccine development.
- What are some key hypotheses that contributed to the understanding of mRNA?
- Sort the full timeline by "Event type" and look for the group of rows with value "Hypothesis".
- You will see a chronological list of key hypotheses related to mRNA research. This list includes significant scientific propositions that contributed to the understanding of mRNA's role in gene expression, protein synthesis, and potential applications, highlighting important developments in molecular biology over the past century.
- What are the key technological advancements in mRNA research?
- Sort the full timeline by "Event type" and look for the group of rows with value "Technological advancement".
- You'll see a chronological list of key technological advancements in mRNA research, highlighting breakthroughs in mRNA delivery systems, gene therapy, and biotechnology.
- Other events are described under the following types: "Background".
Big picture
| Time period | Development summary | More details |
|---|---|---|
| 1961–1989 | Early research | In 1961, Sydney Brenner and colleagues make a significant discovery by identifying an unstable intermediate molecule called mRNA, which copies genetic information from DNA and guides protein synthesis. This marks a turning point in the understanding of mRNA's pivotal role in protein synthesis. In the same year, other scientists such as Cobb, Gros, Hiatt, Gilbert, Kurland, Jacob, and Monod also contribute to the investigation of mRNA's properties and its involvement in protein synthesis. Throughout the 1960s, scientists continue their research on mRNA to further unravel its functions and mechanisms in the synthesis of proteins.[2] In the 1970s, studies are conducted to explore the means of delivering mRNA into cells.[3] |
| 1990 onwards | The rise: mRNA as a therapeutic agent | During the 1990s, there are advancements in the development of mRNA vaccines for personalized cancer treatment that utilize non-nucleoside modified mRNA. Research into mRNA-based therapies persist, exploring their potential as treatments for cancer, autoimmune disorders, metabolic conditions, and respiratory inflammatory diseases. Moreover, the utilization of mRNA to stimulate cells in producing the desired Cas protein showcases potential benefits for gene editing therapies, including CRISPR-based approaches.[4][5] |
| 2000s–2010s | mRNA as a Potential Therapeutic Approach | Researchers successfully use mRNA to produce proteins in live animals. In the mid-2000s clinical trials using mRNA-based vaccines for cancer and infectious diseases show promising results. In 2009, BioNTech is founded. In 2010, Moderna, another of the pioneering mRNA companies, is founded. Since the 2010s, RNA vaccines and other RNA therapeutics start being considered to be "a new class of drugs."[6] |
| 2020s-present | mRNA Breakthroughs and Advancements | Multiple mRNA-based COVID-19 vaccines, including the Pfizer-BioNTech and Moderna vaccines, are authorized for emergency use, demonstrating the efficacy and safety of mRNA technology. mRNA technology gains widespread recognition and acceptance due to the success of COVID-19 vaccines. Ongoing research focuses on expanding the applications of mRNA technology, including therapeutics for cancer, genetic diseases, and personalized medicine. Advancements are being made to optimize mRNA delivery, stability, and immunogenicity for broader clinical use. |
Full timeline
| Year | Condition (when applicable) | Event type | Details | Location |
|---|---|---|---|---|
| 1909 | Cancer | Hypothesis | German physician Paul Ehrlich suggest that the immune system may suppress tumor development. Today this may be demonstrated through promising applications for synthetic mRNA is immunotherapy for cancer[1] | Germany |
| 1944 | Streptococcus pneumoniae | Discovery | A significant milestone is reached with the publication of Oswald Avery, Colin Munro MacLeod, and Maclyn McCarty's seminal paper. This influential work identifies the "transforming principle" within pneumococcal bacteria as DNA. The publication marks a pivotal moment in establishing the recognition of DNA's genetic role, firmly establishing it as the carrier of genetic information and its fundamental importance in biological processes and inheritance.[7][8][9] | United States (Rockefeller University)[10] |
| 1947 | Hypothesis | French biochemist André Boivin becomes the first person to propose the concept that DNA is responsible for generating RNA, which subsequently leads to protein synthesis. Boivin's pioneering insight would lay the foundation for the understanding of the central dogma of molecular biology, highlighting the flow of genetic information from DNA to RNA to proteins.[7] | France | |
| 1950 (March 1) | Hypothesis | Jeener and Szafarz of the University of Brussels first hypothesize that RNA is synthesized in the cell nucleus and then transferred into the cytoplasm, where it was aggregating with other molecules.[11][12] "The first suggestion that small RNA molecules move from the nucleus to the cytoplasm and associate with ribosomes where they drive protein synthesis was made by Raymond Jeener, in 1950."[7] | Belgium | |
| 1952 (September 1) | Hypothesis | American biochemist Alexander Dounce, of Rochester Medical School, proposes a biochemical model of how protein synthesis occurs on an RNA molecule, not on DNA.[13] Although the model is wrong, Dounce hypothesises that “the specific arrangement of amino acid residues in a given peptide chain is derived from the specific arrangement of nucleotide residues in a corresponding specific nucleic acid molecule”.[7] | United States | |
| 1952–1954 | Discovery | French biochemist Jacques Monod's research team and later American biochemist Arthur Pardee conduct experiments that reveal an interesting connection between RNA synthesis and protein synthesis in mutant bacteria. Their findings show that the production of β-galactosidase, an enzyme, was dependent on the presence of a specific RNA nucleotide called uracil. This discovery suggests that RNA synthesis plays a vital role in the process of protein synthesis. The interpretation shared by Crick and the researchers is that the presence of uracil indicates turnover of RNA molecules in the cytoplasm, implying that some RNA molecules undergo degradation and replacement.[14][15][7] | ||
| 1953 | Discovery | James Watson and Francis Crick propose the model of the DNA double helix. After this, a new question would arise in the scientific community: how is information encoded by the DNA and how is it translated?[5] The mRNA molecule would be originally discovered as a result of scientists' search to understand the molecular mechanism by which DNA directs the formation of proteins within a cell, a search that would begin as soon as DNA was cracked by Watson and Crick.[2] | United Kingdom | |
| 1953 | Discovery | Al Hershey and his research team demonstrate that following phage infection, bacteria exhibits a significant increase in the production of a specific type of RNA, which is synthesized at a high rate but also degraded quickly. However, it remains unclear whether this phenomenon is a result of the infection itself and could be considered a pathological consequence.[7][16] | ||
| 1956 | Escherichia coli | Discovery | Elliot Volkin and Lazarus Astrachan at Oak Ridge National Laboratory discover a 'DNA-like RNA' substance that does not resemble previously found types of RNA. Volkin and Astrachan notice it after infecting Escherichia coli with Enterobacteria phage T2 and then exposing the culture to radioactive Phosphorus-32 for a few minutes. Importantly the substance would appear to help the bacteria's cell machinery switch from making its own proteins to those that were characteristic of the virus.[2][7] | United States |
| 1957 | Discovery | The recognition that genes generate a messenger molecule is first realized in Paris when Arthur Pardee visits the Institut Pasteur during his sabbatical. This pivotal observation marks a significant turning point in molecular biology, as it reveals the concept of gene expression through the production of a messenger molecule[17][7][18] | France (Pasteur Institute) | |
| 1958 | Nomenclature | RNA-rich microsomal particles are baptised "ribosomes", during informal discussions at a conference. This term is coined to describe these cellular structures, which play a crucial role in protein synthesis. Ribosomes are composed of ribosomal RNA (rRNA) and proteins, and they function as the sites where mRNA is translated into proteins. The naming and subsequent research into ribosomes significantly advances our understanding of cellular processes and the molecular machinery involved in translating genetic information into functional proteins. This breakthrough lays the foundation for further studies in molecular biology and genetics.[7] | ||
| 1958 (September 29) | Experiment | E. Volkin and L. Astrachan conduct research that reveals important findings about the relationship between RNA and DNA. Their experiments demonstrate that after phage infection in bacteria, radioactive RNA appears rapidly. However, when the radioactive isotope is added later, more radioactivity is observed in DNA compared to RNA. This leads them to speculate about the potential role of RNA as a precursor to DNA synthesis.[19][20][7] | ||
| 1960 | Hypothesis | The idea of mRNA is first conceived by Sydney Brenner and Francis Crick on 15 April 1960 at King's College, Cambridge, while François Jacob tells them about a recent experiment conducted by Arthur Pardee, himself, and Jacques Monod.[7] | United Kingdom | |
| 1960 | Discovery | Masayasu Nomura, Benjamin Hall, and Sol Spiegelman make important progress in the study of RNA synthesis after phage infection. They build upon the previous work of Volkin and Astrachan and identify two distinct forms of RNA: one present in the ribosomal fraction and the other in soluble RNA. The researchers propose two possible interpretations for the soluble RNA fraction. It could be a precursor of ribosomal RNA or its breakdown product, or it might be involved in the amino acid acceptance process similar to Crick's adaptor molecule concept. Their findings shed further light on the intricate mechanisms of RNA function and its role in protein synthesis. [21][7] | ||
| 1960 (December) | Publication | American scientists Sol Spiegelman and Benjamin Hall publish an article showing that in enterobacteria phage T2, DNA and transitory RNA show sequence complementarity and would hybridize.[7][22] The invention of DNA-RNA hybridization by Hall and Spiegelman would have a powerful impact on the theory and discourse of molecular biology.[23] | United States | |
| 1961 | Discovery | Aronson and McCarthy describe RNA molecules of similar size that were attached to ribosomes. This discovery contributes to the understanding of messenger RNA (mRNA) as a key player in protein synthesis. By identifying that these RNA molecules are associated with ribosomes, the cellular structures responsible for translating genetic information into proteins, Aronson and McCarthy provide important insights into the role of mRNA in conveying genetic instructions from DNA to the ribosome, where proteins are assembled. This work is foundational in establishing the concept of mRNA as a crucial intermediary in the process of gene expression.[24] | ||
| 1961 (March) | Publication | American biochemist and geneticist Marshall Warren Nirenberg and his post-doctoral researcher, J. Heinrich Matthaei, publish an article in the journal Biochemical and Biophysical Research Communications. The article documents their cell-free protein synthesis system, highlighting the requirement for both ribosomal RNA and soluble RNA for successful protein synthesis. They find that soluble RNA alone is insufficient to drive the process. Their attempt to fractionate the ribosomal RNA indicates that the biological activity responsible for protein synthesis was associated with a fraction that sedimented approximately three times faster than soluble RNA. This research would shed light on the critical role of ribosomal RNA in the process of protein synthesis.[25][7] | ||
| 1961 | Discovery | A groundbreaking discovery is made with the isolation and characterization of messenger RNA (mRNA). This discovery, published in two articles in Nature, highlights the role of mRNA as an intermediary molecule in protein synthesis. The researchers, including Sydney Brenner, François Jacob, and Jim Watson, find that mRNA carries genetic information from DNA to ribosomes for protein production. This breakthrough revolutionized the understanding of gene function and laid the foundation for subsequent research in molecular biology.[26][27][11][5][2] | ||
| 1961 | Misinterpretation of gene composition | Publication | R.V. Eck publishes a paper in Nature leaving the door open to the possibility that genes are made of proteins, not DNA. This context is essential for understanding the significance of mRNA's discovery as the intermediary in gene expression, confirming DNA's role over proteins.[28][7] | |
| 1961 | Discovery | In what would be known as the Nirenberg and Matthaei experiment, Marshall Warren Nirenberg and J. Heinrich Matthaei decipher the first codon, UUU, as coding for the amino acid phenylalanine. This pivotal discovery marks a significant milestone in molecular biology, providing the first insights into the genetic code that translates the information encoded in mRNA into proteins. By using a synthetic RNA composed of uracil nucleotides, they demonstrate how specific sequences of nucleotides correspond to particular amino acids, ultimately leading to the synthesis of proteins. This work lays the foundation for understanding the genetic code and the process of translation, profoundly impacting genetics and biochemistry.[29][30][31][32] | United States (National Institutes of Health) | |
| 1963 | Viral infection response | Research | Isaacs et al. demonstrate that viral nucleic acids could induce the production of interferon in infected chick, rabbit, and mouse cells. This finding is significant because it shows that the presence of viral nucleic acids—considered foreign by the cell—triggers an immune response, specifically the production of interferon, a protein that plays a crucial role in antiviral defense. The study provides early evidence of how cells recognize and respond to foreign genetic material, laying the foundation for understanding innate immune responses to viral infections. This research also contributes to the broader understanding of how cells detect and react to pathogens at the molecular level.[33][5] | |
| 1965 | Discovery | Marshall W. Nirenberg and Philip Leder complete the genetic code by mapping specific codons to amino acids. This achievement is a critical milestone in molecular biology, providing the foundation for understanding how DNA sequences encode proteins. Their work elucidate the universal language of life, revealing the correspondence between nucleotide triplets (codons) in mRNA and the amino acids they specify during protein synthesis. Nirenberg and Leder's groundbreaking research lay the groundwork for deciphering the genetic information encoded in DNA and paved the way for advancements in genetics, biotechnology, and medicine.[34] | United States | |
| 1965 | October 14 | Recognition | The Nobel Prize in Physiology or Medicine is awarded to François Jacob, André Lwoff and Jacques Monod from Pasteur Institute for the elucidation of the nature of mRNA.[2] | France |
| 1969 | Experiment | Raymond Lockard and Jerry Lingrel from the University of Cincinnati manage to isolate mRNA from a rabbit and introducing it into mouse lymphocytes. This experiment demonstrates that the introduced mRNA could stimulate the production of a specific protein in the lymphocytes. This finding marks a significant step in understanding the role and potential of mRNA in gene expression and protein synthesis.[5][2] | United States (University of Cincinnati) | |
| 1970 | Discovery | Significant advancements are made in understanding the role of transfer RNA (tRNA) in protein synthesis. Researchers elucidate how tRNA facilitates translation, a key step in the process of converting genetic information from messenger RNA (mRNA) into proteins. tRNA molecules act as adaptors, bringing specific amino acids to the ribosome, the cellular machinery responsible for protein assembly. This discovery clarifies how genetic instructions are translated into functional proteins and contributes to the broader understanding of molecular biology. The insights gained from studying tRNA would have profound implications for genetics, biochemistry, and medicine. | ||
| 1973 | Discovery | Canadian physician Ralph M. Steinman and Zanvil A. Cohn at Rockefeller University discover dendritic cells, a type of immune cell found in the bloodstream that helps present antigens to the immune system to activate the T cell response to destroy it. Many of the mRNA cancer vaccines target dendritic cells.[35][2] | United States | |
| 1974 | Discovery | Yasuhiro Furuichi from the National Institute of Genetics reports a key discovery regarding mRNA synthesis, when he finds that during the initial stage of transcription of the double-stranded RNA genome, mRNA synthesis could be activated by a specific nucleotide. This finding sheds light on the mechanisms involved in the transcription process and provides insights into the regulation of mRNA synthesis.[2] | Japan (National Institute of Genetics) | |
| 1975 | Discovery | Yasuhiro Furuichi and A. J. Shatkin at AGENE Research Institute in Kamakura, Japan, discover a unique 'cap' structure at the tip end of mRNA. Known as the 5' cap, this structure plays a crucial role in mRNA stability, transport, and translation initiation. The discovery of the mRNA cap provides valuable insights into the molecular mechanisms of gene expression and paved the way for further research in the field of molecular biology.[36][2][2] | Japan (AGENE Research Institute) | |
| 1977 | Discovery | Richard J. Roberts and Phillip A. Sharp independently discover that genes can be discontinuous, existing as separate segments in DNA. This would lead to the understanding of split genes and RNA splicing, revealing the presence of introns and exons. Introns are non-coding sequences spliced out of RNA before translation, while exons are the coding sequences that form mature RNA. This discovery alters the understanding of gene development during evolution, showing that split genes can generate new genes or proteins and that a single gene can encode multiple proteins.[37][38][39][40] | ||
| 1978 | Technological advancement | Liposomes are first utilized as a method for delivering mRNA to eukaryotic cells, marking an early step in the development of mRNA-based technologies. Liposomes, which are small vesicles composed of lipid bilayers, provide a protective environment for mRNA, facilitating its entry into cells without degradation. This approach lays the groundwork for future advancements in mRNA delivery systems. By the end of the 1980s, a significant development would occur with the description and commercialization of DOTMA (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride), a cationic liposome mRNA delivery system. DOTMA would become a widely used tool in molecular biology, enhancing the efficiency and reliability of mRNA delivery for research and therapeutic purposes.[5] | ||
| 1978 (August) | Experiment | Dimitriadis, Ostro, Giacomoni, Lavelle, Paxton, and Dray conduct an important experiment at the National Institute for Medical Research and the University of Illinois. They successfully produce proteins in mouse and human cells through the delivery of mRNA that was packaged in a liposome. This groundbreaking approach demonstrates the potential of using mRNA as a therapeutic tool for protein production, opening new avenues for research and development in the field of molecular medicine.[41][2][42] | United Kingdom (National Institute for Medical Research), United States (University of Illinois) | |
| 1978 | Discovery | Thomas Cech and Sidney Altman independently discover ribozymes, a finding that demonstrates RNA molecules can catalyze chemical reactions, a role previously thought to be exclusive to proteins. This discovery challenges the central dogma of molecular biology, which at the time holds that proteins are the sole enzymes. The understanding of ribozymes provides crucial insights into RNA's versatility, laying the foundation for mRNA research. It opens the possibility that RNA can not only carry genetic information but also play active roles in cellular processes, ultimately leading to the development of mRNA-based therapies and vaccines. The 1989 Nobel Prize in Chemistry would be awarded to Tom Cech and Sidney Altman for their independent discoveries of ribozymes.[43] | United States | |
| 1983 | Discovery | Jeffrey et al. report that the distribution of actin mRNA in ascidian oocytes and embryos exhibit an asymmetrical pattern. This finding marks the initial identification of key components in understanding the phenomenon.[11] | ||
| 1983 | Discovery | Researchers make a significant discovery by identifying two key features of mRNA: the 5' cap and the 3' polyadenylation. The 5' cap, a modified guanine nucleotide added to the beginning of the mRNA molecule, and the 3' polyadenylation, a tail of adenine nucleotides added to the end, are found to be essential for mRNA stability and the initiation of translation. These modifications protect mRNA from degradation, assist in the export of mRNA from the nucleus, and are crucial for the efficient initiation of protein synthesis. This discovery is pivotal in understanding the regulation of gene expression at the mRNA level. | ||
| 1984 | Technological advancement | Philipp Felgner, working at Syntex Research, manages to synthesize the first cationic (positively charged) lipid. The use of cationic lipids provide a promising approach for delivering therapeutic drugs and genetic material into cells more efficiently. This breakthrough marks an important step forward in the development of novel delivery systems and paves the way for further advancements in drug and gene therapy research.[2] | ||
| 1984 | Technological advancement | Developmental biologists Paul Krieg and Douglas Melton from Harvard University demonstrate the production of abundant and functional mRNA in the laboratory. They achieve this by utilizing an RNA-synthesis enzyme derived from the vaccinia virus and employing various techniques. Their research would pave the way for the generation of significant quantities of biologically active mRNA, contributing to advancements in the field of molecular biology.[44][5] | United States (Harvard University) | |
| 1985 | Technological advancement | S. Tabor and C. C. Richardson develop an upgraded version of the T7 polymerase-promoter complex, allowing for precise regulation of gene expression. This innovative approach remains in use today. These groundbreaking experiments initiated a continuous progression of practical research focused on mRNA delivery and commercialization, leading to significant advancements in the field.[45][5] | ||
| 1987 | Experiment | American physician and biochemist Robert W. Malone conducts an experiment that demonstrates the ability to induce cells to produce luciferase, a light-producing protein. He achieves this by injecting the cells with mRNA that he had prepared using the technique developed by Krieg and Melton, combined with cationic lipids provided by Felgner. This experiment, conducted at the Salk Institute for Biological Sciences in collaboration with Felgner and Verma, shows that mRNA encapsulated into liposomes made with cationic lipids could effectively stimulate protein production when injected into mouse cells. This breakthrough provides further evidence for the potential of mRNA-based therapies and marks an important milestone in the development of mRNA delivery systems for protein expression.[46] | United States (Salk Institute) | |
| 1989 | Technological advancement | The concept of using mRNA as a therapeutic agent is first proposed following the development of a broadly applicable in vitro transfection technique. This technological advancement enables scientists to introduce mRNA into cells in a controlled laboratory setting, laying the groundwork for mRNA-based therapies. The in vitro transfection method allows for the efficient delivery and expression of mRNA within cells, demonstrating its potential to direct the production of therapeutic proteins. This breakthrough marks a pivotal moment in biotechnology, opening the door to innovative approaches in treating diseases by harnessing the power of mRNA.[47] | ||
| 1989 | Experiment | Dimitriadis conducts an experiment using liposomes for the delivery of mRNA to eukaryotic cells. This experiment is a significant step in developing methods to effectively introduce mRNA into cells, a critical challenge for mRNA-based therapies. The use of liposomes—spherical vesicles with lipid bilayers—allows for the encapsulation and protection of mRNA, facilitating its entry into cells. By the end of the following decade, this research would lead to the description and commercialization of DOTMA, a cationic liposome mRNA delivery system. DOTMA represents an important advancement in the field, enabling more efficient and targeted delivery of mRNA for therapeutic purposes.[5] | ||
| 1990 (January 1) | Experiment | An experiment by Kariko et al. at University of Pennsylvania shows that mRNA can be used to get cells to produce protein that inhibits blood clot formation.[2] | United States | |
| 1990 (March 23) | Discovery | A study reports that when naked mRNA and naked DNA were directly injected into the skeletal muscle of mice, it results in the production of proteins. The research was conducted by Wolff, Malone, Williams, Chong, Acsadi, and Jani from the University of Wisconsin, Salk Institute for Biological Sciences, and Vical.[2][48] | United States (University of Wisconsin, Salk Institute) | |
| 1990 | Discovery | Following the initial endeavors in DNA-based gene therapy, the viability of mRNA as a genetic vector is demonstrated. This approach holds theoretical appeal due to the non-integration of mRNA into the genome, its immediate availability for protein translation, and its transient nature that is advantageous for certain applications. Wolff et al., in a notable study, inject naked mRNA encoding chloramphenicol acetyltransferase into the skeletal muscle of mice and successfully observed the expression of specific proteins. This marks the first published account of the effective utilization of in vitro transcribed (IVT) mRNA in animals, where injected reporter gene mRNAs results in detectable protein production.[1][49][50] | ||
| 1990 | Discovery | The concept of mRNA as a therapeutic agent is established by Wolff J. and colleagues, when they conduct an experiment demonstrating the in-vivo expression of mRNA. The researchers inject naked RNA directly into the muscles of mice, providing evidence of direct gene transfer in living organisms. This study lays the foundation for the use of mRNA as a potential tool for therapeutic applications.[5] | ||
| 1991 | Cancer | Therapeutic development | mRNA is proposed as a potential active pharmaceutical ingredient for the treatment of cancer. This idea emerges from the growing understanding of mRNA's role in protein synthesis and its potential to be harnessed for therapeutic purposes. The concept involves using mRNA to instruct cells to produce proteins that can stimulate an immune response against cancer cells or directly target and attack tumor cells. This proposal marks an early recognition of mRNA's potential in cancer therapy, paving the way for subsequent developments in the use of mRNA for targeted treatments and immunotherapies in oncology.[51] | |
| 1992 (February 21) | Diabetes | Therapeutic development | Jirikowski et al. at The Scripps Research Institute Diabetes demonstrate that mRNA encoding for the hormone vasopressin directly injected into the brains of rats could temporarily relieve diabetes for up to 5 days.[2][5][52] | United States |
| 1992 | Diabetes | Therapeutic development | A significant milestone is reached in the utilization of mRNA as a genetic vector. Prior to this, the theoretical advantages of mRNA, such as its non-integration into the genome, immediate availability for protein translation, and transient signaling, had been recognized. The practical demonstration of mRNA's capabilities occurrs in 1990 when Wolff et al. inject naked mRNA encoding chloramphenicol acetyl transferase into the skeletal muscle of mice, resulting in the targeted expression of the specific protein. Building upon this success, a subsequent study in 1992 focuses on the treatment of chronic diabetes insipidus in Brattleboro rats. In this experiment, naked, synthetic mRNA encoding arginine vasopressin was injected into the rats' hypothalami, leading to a successful cure of the condition. These findings exemplify the potential of mRNA as a genetic tool, showcasing its ability to deliver therapeutic benefits in gene therapy approaches.[1] | |
| 1992 | Diabetes | Therapeutic development | A study demonstrates that administration of vasopressin-encoding mRNA in the hypothalamus could elicit a physiological response in rats. This research highlights the potential of using mRNA as a therapeutic tool by showing that it could effectively instruct cells to produce a specific protein—in this case, vasopressin, a hormone involved in regulating water balance and blood pressure. This study lays the groundwork for further exploration into mRNA-based therapies, suggesting that mRNA can be used to deliver therapeutic proteins and address various medical conditions, including endocrine disorders and potentially diabetes.[49][53] | |
| 1993 (April 20) | Publication | Joel G. Belasco and George Brawerman publish the book Control of Messenger RNA Stability. This work provides a comprehensive examination of the mechanisms regulating the stability of mRNA molecules within cells. The book discusses how the degradation of mRNA influences gene expression and cellular responses, emphasizing the role of various regulatory elements and processes in controlling mRNA longevity. This publication is a key resource for understanding the dynamic regulation of mRNA stability, which is crucial for gene expression control and has implications for fields such as molecular biology, genetics, and therapeutic development.[54] | United States (University of Michigan) | |
| 1993 (July) | Vaccine development | The first evidence suggesting that mRNA could serve as a potential method for vaccines occurs when researchers Martinon, Krishnan, Lenzen, Magne, Gomard, and Guillet from INSERM present compelling findings supporting the notion that mRNA could be utilized as a viable approach for vaccine development. This discovery opens up new possibilities for utilizing mRNA as a tool in immunization strategies.[2] | France (Inserm) | |
| 1993 | Influenza | Discovery | Martinon et al. show that subcutaneous injection of liposome-encapsidated mRNA encoding the influenza virus nucleoprotein induces anti-influenza cytotoxic T lymphocytes.[1] | |
| 1994 | Hypothesis | Kozak proposes a hypothesis regarding the structural characteristics of the 5' untranslated region (UTR) of mRNA molecules. According to his postulation, these structures could function as regulatory sequences that facilitate translation by enabling proteins to bind and induce conformational changes downstream. This concept suggest that the specific arrangement of nucleotides in the 5' UTR could play a role in modulating the efficiency of protein synthesis. Kozak's hypothesis provide valuable insights into the mechanisms underlying translational control and the potential regulatory functions of mRNA structures.[55] | ||
| 1995 | Publication | Conry et al. publish study on development of mRNA transcripts encoding luciferase and human carcinoembryonic antigen (CEA) as a vaccine vector. The modified mRNA constructs successfully expresses CEA in mouse fibroblasts in vitro and luciferase in vivo after injection. The researchers immunized mice with the mRNA transcripts encoding CEA, resulting in an immune response with anti-CEA antibodies detected in five out of seven immunized mice. This approach shows promise for inducing immune responses against potential cancer-related proteins.[5][56] | ||
| 1995 | Publication | Ian Maclachlan and Pieter Cullis publish a study on the development of a lipid nanoparticle system for the delivery of drugs and gene therapy. This research, conducted at Protiva Biotherapeutics and Inex Pharmaceuticals, introduces a novel approach that utilized lipid nanoparticles to effectively deliver therapeutic agents and genetic material. The study opens up new possibilities in the field of drug delivery and gene therapy by providing a promising platform for targeted and efficient delivery of treatments.[57][5] | ||
| 1996 | Cancer | Experiment | Gilboa and colleagues demonstrate the potential to elicit robust immune responses against tumors in mice by utilizing dendritic cells that were modified with mRNA encoding surface receptors found on the tumors.[2] | |
| 1996 (August ) | Cancer | Experiment | Boczkowski et al. at Duke University show that dendritic cells modified with mRNA elicit strong immune response against tumours in mice. This experiment shows that mRNA can be used to engineer dendritic cells—key players in the immune system—to produce tumor-specific antigens. When these modified cells are introduced into mice, they trigger an effective immune response targeting the tumors. This groundbreaking work provides early evidence of mRNA’s potential in cancer immunotherapy, paving the way for further development of mRNA-based approaches to enhance the immune system's ability to fight cancer.[2] | United States |
| 1996 | Discovery | RNA interference (RNAi) is discovered, revealing a mechanism in which small RNA molecules regulate gene expression. This groundbreaking discovery would revolutionize molecular biology and earn the Nobel Prize in Physiology or Medicine in 2006 for its discoverers Andrew Fire and Craig Mello. RNAi would since become a crucial tool in scientific research, enabling precise control over gene expression and offering promising applications in therapeutics, agriculture, and biotechnology. | ||
| 1997 | Discovery | Canadian American biologist Jack W. Szostak and Richard W. Roberts show that fusions between a synthetic mRNA and its encoded myc epitope could be enriched from a pool of random sequence mRNA-polypeptide fusions by immunoprecipitation.[58] | ||
| 1997 | Organization | Merix Bioscience, founded as a Duke University spin-out, is established to develop mRNA for cancer vaccines. It becomes the first-ever mRNA company, marking a significant milestone in the field of mRNA therapeutics. The company later changes its name to Argos Therapeutics in 2004.[5][59][60][2] | United States | |
| 1997 (October) | Publication | Joel D. Richter publishes the book mRNA Formation and Function. The text delves into the detailed processes of mRNA synthesis, processing, and its crucial role in gene expression. Covering topics such as transcription, splicing, and translation, this work provides comprehensive insights into mRNA's function within cells. The book is a significant resource for understanding the complexities of mRNA biology and its applications in molecular genetics and biotechnology.[61] | ||
| 1998 | Discovery | A small interfering RNA (siRNA) or micro RNA (miRNA), is discovered as a class of naturally occurring RNA. This discovery, made by MK and Kostas, reveals that siRNA and miRNA play a crucial role in the post-transcriptional machinery of cells. By targeting specific mRNAs, siRNA and miRNA can form double-stranded RNA molecules that prompt rapid degradation (known as gene silencing). Consequently, this mechanism offers the potential to regulate gene expression at a post-transcriptional level. The utilization of siRNA and miRNA holds promise in the development of treatments for HIV, cancer, and melanoma.[5] | ||
| 2000 (January) | Experiment | A significant advancement is made in the field of vaccination when Hoerr, Obst, Ramemenseee, and Jung from the University of Tübingen demonstrate the efficacy of freshly synthesized naked RNA and protamine-protected RNA as viable tools for vaccination. This finding indicates that these RNA molecules could serve as effective agents in vaccine development.[2] | ||
| 2000 | Organization | CureVac, a spin-out company, is established with the purpose of developing mRNA for vaccines. Ingmar Hoerr, the founder of CureVac, plays a crucial role in this endeavor. CureVac AG emerges as the pioneer company to successfully apply mRNA in the field of medicine, marking a significant milestone in the utilization of mRNA technology for medical purposes.[2][59] | Germany | |
| 2000 (October) | Discovery | A major discovery is made at the University of Pennsylvania by Drew Weissman, H. Ni, D. Scales, Dude, Capodici, McGibney, Abdool, SN Isaacs, Cannon, and Kariko. They report that mRNA encoding for HIV is able to activate a powerful immune response in T cells. This finding opens up new possibilities for the development of mRNA-based vaccines targeting HIV, with the potential to stimulate a robust immune defense against the virus.[2] | United States (University of Pennsylvania) | |
| 2004 (March) | Discovery | A report indicates that mRNA can activate a series of Toll-like receptors, which are signaling receptors of the innate immune system. This research was conducted by Kariko, Houping Ni, Capodici, Lamphier, and Drew Weissman from the University of Pennsylvania.[2] | ||
| 2005 (August) | Discovery | Research team led by Katalin Karikó at University of Pennsylvania finds that RNA is rendered invisible to immune system by replacing its nucleoside uridine with pseudouridine. This modification prevents the immune system from recognizing and attacking the RNA, which is a significant obstacle in the development of RNA-based therapies. Karikó's work is pivotal in overcoming this challenge, paving the way for the development of mRNA vaccines and therapeutics. This discovery would have far-reaching implications in medical research and biotechnology.[62][2] | United States (University of Pennsylvania) | |
| 2005 (October 19) | Therapeutic development | MacLachlan and Cullis from Protiva Biotherapeutics and Inex Pharmaceuticals publish an important development in drug delivery and gene therapy. They introduce a lipid nanoparticle system designed for the efficient delivery of drugs and gene therapies. This innovative approach utilizes lipid-based nanoparticles to encapsulate and transport therapeutic agents, offering improved targeting and delivery capabilities.[2] | ||
| 2006 | Organization | A spin-out company called RNARx is established with the aim of commercializing modified mRNA for the treatment of anaemia. This venture is founded by Kariko and Drew Weissman, who played a crucial role in the development and modification of mRNA technology. The company's focus is to leverage the potential of modified mRNA to address anaemia and explore its therapeutic applications in this specific area. The establishment of RNARx marks a significant step towards translating mRNA research into real-world medical solutions for anaemia treatment.[2] | ||
| 2006 (March 26) | Experiment | A significant breakthrough is achieved in the field of gene therapy through monkey studies. Researchers conduct experiments using a lipid nanoparticle system to successfully deliver RNA and silence disease-causing genes. This achievement demonstrates the potential of this novel approach for gene therapy. The successful delivery of RNA using the lipid nanoparticle system in monkey studies would pave the way for future advancements in the field of RNA-based therapeutics. The research is conducted under the auspices of Protiva Biotherapeutics, a company at the forefront of developing innovative solutions in the field of gene therapy.[2] | ||
| 2006 (December 15) | Technological advancement | A method for producing mRNA with enhanced stability and translational efficiency is published. The method proposes innovative techniques to improve the characteristics of mRNA, making it more stable and efficient in protein translation. This advancement would opened up new possibilities for mRNA-based applications and further contribute to the development of RNA-based therapies and biotechnological tools.[2] | Germany (Johannes Gutenberg University) | |
| 2008 | Hypothesis | The initial concept of delivering self-amplifying RepRNA vaccines using synthetic, biodegradable particles is introduced. Since then, the approach of complexing the RNA for targeted delivery to dendritic cells (DCs) would demonstrate its potential through various methods such as polysaccharide, polyplex, and lipoplex formulations.[63][64] | ||
| 2008 | Organization | Biopharmaceutical New Technologies (BioNTech) is established with the aim of utilizing mRNA for the development of personalized cancer immunotherapies. The founders recognize the potential of mRNA technology in the field of cancer treatment and seek to harness its capabilities for more accurate and individualized immunotherapy approaches. The establishment of BioNTech marks a significant event in the advancement of mRNA-based therapies, with a focus on tailoring treatments to the specific needs of cancer patients. Since its inception, BioNTech would be dedicated to pushing the boundaries of immunotherapy and would play a role in the development of innovative mRNA-based cancer treatments.[2][59] | Germany | |
| 2009 | Metastatic melanoma | Clinical trial | A groundbreaking trial is conducted on mRNA-based vaccines for cancer immunotherapy in individuals with metastatic melanoma. The trial demonstrates the potential of mRNA technology to stimulate the immune system and generate vaccine-specific T cells targeted against melanoma. The results show a significant increase in the number of T cells directed against melanoma, indicating the vaccines' ability to elicit an immune response. This trial marks a significant advancement in the development of mRNA-based vaccines for cancer treatment and provides promising evidence for their use in cancer immunotherapy.[5] | |
| 2010 | Technological advancement | Derrick Rossi, a researcher at Harvard Medical School, expands upon Shinya Yamanaka's breakthrough in inducing pluripotency and utilizes RNA to reprogram adult cells into embryonic stem cells. This significant development paves the way for the establishment of Moderna Therapeutics, as it harnesses the potential of RNA technology for various applications in medicine and therapeutics.[5][65] | United States (Harvard Medical School) | |
| 2010 | Technological advancement | Eduard Yakubov et al. develop a method to reprogram human fibroblasts into induced pluripotent cells (iPS) using mRNA derived from specific transcription factors. Their approach aim to reduce the risks associated with DNA integration by traditional reprogramming methods. By transfecting the cells with RNA synthesized from cDNA of the transcription factors, the researchers observed successful intracellular protein expression and nuclear localization. Through multiple transfections, iPS colonies expressing embryonic stem cell markers were generated. Importantly, this RNA transfection approach eliminates the need for DNA integration and holds promise as a potential alternative to DNA vectors in iPS generation..[66][5] | Israel (Weizmann Institute of Science) | |
| 2010 (November) | Organization | Moderna Therapeutics is established with the objective of commercializing modified mRNA vaccines and therapeutics. Founded by Rossi, Kernneth Chien, and Robert Langer, Moderna enters the scene as the final member of the "mRNA Big Three" companies to be established. Despite being the last to emerge, Moderna would quickly gain significant recognition and is highly valued, surpassing the other two companies in the field. With a focus on mRNA therapies, Moderna would make notable progress in the development of innovative treatments using mRNA as a therapeutic agent.[59][5] | ||
| 2010 (November 5) | Technological advancement | A team led by Luigi Warren, Philip Manos, and Chad Cowan report the successful transformation of skin cells into pluripotent stem cells using modified mRNA. The researchers utilized modified mRNA to reprogram the skin cells into a pluripotent state, capable of differentiating into various cell types.[5] | United States (Harvard University) | |
| 2011 | Partnership | CureVac establishes a partnership with Sanofi Pasteur, the vaccines division of Sanofi. This collaboration focuses on developing mRNA-based vaccines, combining CureVac’s expertise in mRNA technology with Sanofi Pasteur's experience in vaccine development. The partnership aims to explore new approaches to vaccine design and production, leveraging mRNA’s potential to create more effective and flexible vaccines for various infectious diseases. This alliance is part of a broader trend in the pharmaceutical industry to invest in innovative technologies for advancing public health. | ||
| 2012 | Discovery | Breakthroughs in genetic engineering are made by two separate teams led by Jennifer Doudna and Emmanuelle Charpentier. Their work focuses on utilizing CRISPR for precise genome editing. The CRISPR system, consisting of the Cas9 enzyme and a guide RNA, is found to target specific sequences in the genome. Plasmids or viral vectors are used to introduce these components into target cells. However, the sustained presence of Cas9-encoding plasmid DNA can lead to unintended modifications in non-targeted regions of the genome. These discoveries revolutionize genetic engineering and offer new opportunities for precise genome editing.[67][5] | ||
| 2013 | Cancer | Organization | RNA Immunotherapies, a Belgian biotechnology company, is founded with the objective of developing immunotherapy for cancer and infectious diseases. The company is established in collaboration with Professor Kris Thielemans, a renowned immunologist from the Free University of Brussels, along with other partners. Their focus is on utilizing their proprietary mRNA TriMix platform for the development of innovative immunotherapies.[59] | Belgium |
| 2013 | Cardiometabolic diseases, cancer | Partnership | Moderna partners with AstraZeneca to develop messenger RNA therapeutics for cardiometabolic diseases and cancer. This collaboration, involving a $240 million upfront payment from AstraZeneca, focuses on a novel treatment approach that enables the body to produce therapeutic proteins in vivo. AstraZeneca gains exclusive rights to develop up to 40 drug products over five years, with Moderna entitled to up to $180 million in milestone payments and royalties. Moderna's technology aims to create innovative drugs for previously undruggable targets, with AstraZeneca leading the clinical development and commercialization efforts.[59][68] | United States, United Kingdom, Sweden |
| 2013 | Influenza | Partnership | CureVac partners with Johnson and Johnson's Janssen Pharmaceuticals to develop a mRNA flu vaccine. This collaboration aims to leverage CureVac's expertise in mRNA technology to create a novel vaccine platform for influenza. The partnership seeks to advance the development of an mRNA vaccine that can potentially offer more effective and adaptable protection against flu viruses compared to traditional vaccine approaches. This initiative underscored the growing interest and investment in mRNA technology as a versatile tool for combating infectious diseases.[59] | |
| 2013 (October) | Cardiovascular diseases | Experiment | A group of researchers from Harvard University, Massachusetts General Hospital, Children's Hospital Boston, Mount Sinai School of Medicine, and Karolinska Institute, conduct a study that demonstrates the potential of modified mRNA in improving heart function in mice. Through their research, they utilized modified mRNA and observed significant improvements in heart function in the mice subjects. The findings of this study highlight the therapeutic efficacy of modified mRNA in enhancing heart function. This discovery would open up promising avenues for further investigations into the use of modified mRNA as a potential treatment for heart-related conditions. The study provides valuable insights and set the stage for future research in the development of novel therapies for cardiovascular diseases.[5] | |
| 2014 | Infectious diseases, Hypersensitivities, Cancer | Clinical trial | Numerous pre-clinical and clinical trials start being carried out to investigate the utilization of mRNA technology in the development of vaccines targeting infectious diseases, hypersensitivities, and cancer. These trials, as documented by Sahin et al. (2014) and Weissman (2015), encompass a range of studies that aim to evaluate the effectiveness and safety of mRNA-based vaccines in combating these conditions.[5] | |
| 2015 (May 11) | Cancer | Partnership | BioNTech announces a partnership agreement with Eli Lilly, with the purpose to advance the development of new tumor immunotherapy treatments, specifically focusing on T-cell receptor (TCR) therapies. The partnership involved a significant financial commitment, with Lilly investing more than $360 million in the collaboration. The objective of the collaboration was to leverage BioNTech's expertise in mRNA technology and Lilly's experience in the development and commercialization of innovative therapies. By combining their resources and knowledge, the two companies aim to accelerate the progress of tumor immunotherapy treatments, with a particular emphasis on TCR therapies.[69][59] | |
| 2015 | Immunity | Discovery | Katalin Karikó and her colleague Drew Weissmann discover a solution to prevent the activation of the immune response triggered by injected mRNA. It is observed that mRNA activates toll-like receptors (TLR) present on immune cells. To address this, Karikó and Weissmann introduced a modification to the RNA by incorporating a naturally occurring modified nucleoside known as pseudouridine.[5] | |
| 2016 | Animal health, Cancer | Partnership | BioNTech establishes collaborations with two major companies. The first is with Bayer, aiming to develop innovative mRNA vaccines and drugs for animal health. This partnership aims to leverage BioNTech's mRNA technology platform to advance veterinary healthcare. In September of the same year, BioNTech joins forces with Genentech, a subsidiary of Roche, to develop an individualized mRNA tumor vaccine. This vaccine is designed to target specific neoantigens, which are unique to each patient's tumor. By utilizing mRNA technology, the goal is to develop personalized cancer immunotherapy that could effectively target and treat tumors based on the patient's specific genetic makeup.[59] | |
| 2016 | Technological advancement | The development of CRISPR-Cas9 technology revolutionizes the field of genetic engineering by providing a precise and versatile tool for gene editing. This technology uses a bacterial immune system to make targeted cuts in DNA, allowing scientists to modify specific genes with high accuracy. The advent of CRISPR-Cas9 opens new possibilities for manipulating mRNA by enabling the correction of genetic mutations that cause diseases, optimizing mRNA expression, and studying gene function. Its potential applications extend to treating genetic disorders, advancing research in functional genomics, and developing innovative therapies, including those that leverage mRNA technology.[70] | ||
| 2016 (November) | Genetic disorders | Organization | Translate Bio, a Massachusetts-based company specializing in RNA technology, is officially registered under its original name, RaNA Therapeutics. The company's focus is on developing mRNA-based therapeutics for various diseases, leveraging the potential of RNA to address genetic disorders and other conditions. The rebranding from RaNA Therapeutics to Translate Bio reflects its commitment to advancing RNA science and its applications in medicine. This change marks a significant step in the company's evolution and its continued efforts to harness mRNA technology for innovative therapeutic solutions.[59] | United States |
| 2017–2019 | HIV, Rabies | Scientific advancement | Significant advancements are achieved in the effectiveness of mRNA encoding antibodies against HIV and rabies, as evidenced by studies conducted by Pardi et al., Thran et al., and Schlake et al. in 2019. These studies contribute to the growing understanding of the potential of mRNA-based approaches in combating these infectious diseases.[5] | |
| 2017 | Cancer | Experiment | In a study conducted by Stadler et al., researchers demonstrate the effectiveness of mRNA-encoded bispecific antibodies in eliminating large tumors in mice. By using in vitro-transcribed, pharmacologically optimized mRNA encoding the antibody, they achieved sustained endogenous synthesis of the antibody in the mice. The results show that the mRNA-encoded antibodies are as effective as purified bispecific antibodies in eliminating advanced tumors. This approach offers a potential solution to manufacturing challenges and could expedite the clinical development of novel bispecific antibodies due to the fast production of pharmaceutical mRNA.[5][71] | |
| 2017 (October) | Cancer | Partnership | CureVac enters into a strategic partnership with Eli Lilly to jointly develop and commercialize five cancer vaccine products. This collaboration aims to leverage CureVac's expertise in mRNA-based therapies for cancer treatment. Additionally, in November of the same year, CureVac forms a partnership with CRISPR to advance the development of Cas9 mRNA for in vivo gene editing. These partnerships highlight the direction aimed at advancing innovative mRNA technologies for therapeutic applications in cancer treatment and gene editing.[59] | United States |
| 2018 | Partnership | CureVac and Arcturus form a strategic partnership aimed at collaborating on the identification, development, and commercialization of novel mRNA therapies. This partnership brings together the expertise and resources of both companies to advance the field of mRNA-based therapeutics and explore new treatment possibilities.[59] | ||
| 2019 | Cancer | Partnership | Danish biotechnology company Genmab and CureVac partner to develop mRNA-based antibody therapeutics. The collaboration aims to combine CureVac's mRNA technology with Genmab's antibody expertise to create innovative treatment options for cancer patients. Genmab agrees to provide CureVac with a $10 million upfront payment and make a €20 million equity investment. The companies agree to conduct joint research to identify an initial product candidate, with Genmab taking responsibility for its development and commercialization. The partnership is expected to contribute to advancing mRNA-based antibody therapeutics in the field of cancer treatment.[59][72] | |
| 2019 | HIV | Experiment | A study conducted by Pardi et al. demonstrates the efficacy of nucleoside-modified mRNA in encoding a broadly neutralizing antibody against HIV-1. The researchers generated nucleoside-modified mRNAs encoding the light and heavy chains of the anti-HIV-1 antibody VRC01 and administered them to mice using lipid nanoparticles. The systemic administration of the mRNA resulted in significant levels of the VRC01 antibody in the plasma. Weekly injections of the mRNA maintained sustained antibody levels in mice. Importantly, a single injection of VRC01 mRNA provided protection against intravenous HIV-1 challenge in humanized mice. This study highlights the potential of nucleoside-modified mRNA as a delivery platform for passive immunotherapy against HIV-1 and potentially other diseases.[5][73] | |
| 2019 | Toxins | Therapeutic development | It is reported that mRNA technology could be utilized for the production and administration of antibodies against toxins. In cases where individuals are exposed to venom or toxins, immediate neutralization is crucial. Normally, it takes the body several days or weeks to generate antibodies, but a rapid response is essential in such situations. Traditionally, antibodies against toxins are produced using expensive and less accessible mammalian cell cultures. However, mRNA technology offers a more direct approach for antibody production. These antibodies can be administered through various routes such as intravenous, intradermal, subcutaneous, intramuscular, or intranodal, allowing for a swift response. Therefore, mRNA-mediated neutralization presents a promising and fast-acting alternative to conventional antibody use.[74][5] | |
| 2019 | Experiment | Lindsay et al. conduct a study on mRNA vaccine behavior within the body, focusing on its uptake and expression by immune cells. They observe a strong mRNA signal in the lymphoid node within 4 hours of intramuscular administration. This successful accumulation of mRNA in the lymphoid node highlights the importance of administration routes for vaccine distribution and immune cell uptake. The findings provide valuable insights into mRNA vaccine behavior, emphasizing the role of administration routes in shaping the immune response. Understanding the timing and localization of mRNA uptake in the lymphoid node contributes to optimizing vaccine delivery strategies and enhancing their effectiveness.[5] | United States (Georgia Tech, Emory University) | |
| 2019 (December 6) | Publication | Marlene Oeffinger and Daniel Zenklusen publish The Biology of mRNA: Structure and Function, which provides an in-depth exploration of the biology of messenger RNA (mRNA), focusing on its structure, function, and role in cellular processes. It covers the mechanisms of mRNA synthesis, processing, and translation, as well as its regulatory aspects and implications for gene expression. The book is aimed at researchers and students in molecular biology, genetics, and related fields, offering comprehensive insights into the fundamental aspects of mRNA and its impact on cellular and molecular biology.[75] | ||
| 2019 (December) | COVID-19 | Background | The emergence of the COVID-19 pandemic highlights an urgent global need for new vaccines. The novel coronavirus, SARS-CoV-2, quickly spreads across the world, causing widespread illness and significant disruptions. The rapid increase in cases and severity of the disease spurs unprecedented efforts in vaccine research and development. Scientists and pharmaceutical companies worldwide focus on creating effective vaccines to curb the spread of the virus and protect public health. This urgent need for vaccines leads to accelerated research, collaboration, and the eventual development and approval of several COVID-19 vaccines within a remarkably short timeframe. | Worldwide |
| 2020 | COVID-19 | Vaccine development | Pfizer and BioNTech announce their collaboration to co-develop a potential mRNA-based vaccine, called BNT162, for preventing COVID-19 infection. The companies signed a letter of intent and a Material Transfer and Collaboration Agreement to begin working together immediately. The collaboration aims to accelerate the development of the vaccine. This partnership builds on their previous agreement to develop mRNA-based vaccines for influenza.[76][59] | United States, Germany |
| 2020 (July) | Infectious diseases | Partnership | GlaxoSmithKline (GSK) and CureVac announce a strategic collaboration agreement for the development of mRNA-based vaccines and monoclonal antibodies (mAbs) targeting infectious diseases. The partnership aims to leverage CureVac's expertise in mRNA technology and GSK's scientific leadership in vaccines and mAbs. The companies agree to work on up to five mRNA-based vaccines and mAbs, with a focus on infectious disease pathogens. GSK agrees to invest £130 million (€150 million) in CureVac, representing a 10% stake, and provide an upfront payment of £104 million (€120 million). CureVac would be responsible for preclinical and clinical development through Phase 1 trials, after which GSK will take over further development and commercialization. The collaboration is expected to contribute to advancing mRNA-based vaccine and treatment technologies and improving responses to future pandemics.[59][77] | United Kingdom, Germany |
| 2020 (December) | COVID-19 | Vaccine development | Pfizer–BioNTech and Moderna obtain authorization for their mRNA-based COVID-19 vaccines. "In 2020, the FDA approved the first mRNA-based vaccines against an infectious disease SARS-CoV-2. This was only made possible by decades of research on mRNA-based therapeutics."[5] | |
| 2020 (December 11) | COVID-19 | Vaccine development | The U.S. FDA approves the first mRNA vaccine for COVID-19 for emergency use, by BioNTech and Pfizer. This landmark approval marks a significant advancement in the pandemic response, introducing a new vaccine technology based on messenger RNA (mRNA). The Pfizer-BioNTech vaccine, branded as Comirnaty, works by instructing cells to produce a protein similar to the spike protein of the SARS-CoV-2 virus, thereby triggering an immune response. This authorization is a pivotal moment in the global effort to control COVID-19 and laid the groundwork for subsequent vaccine approvals.[2][78] | United States |
| 2020 (December 18) | COVID-19 | Vaccine development | The U.S. FDA approves second mRNA vaccine for COVID-19, by Moderna. This vaccine, known as the Moderna COVID-19 vaccine, follows the earlier approval of the Pfizer-BioNTech vaccine, marking a significant milestone in the fight against the pandemic. The mRNA technology used in these vaccines represents a novel approach, instructing cells to produce a protein that triggers an immune response. This authorization expands the options available for vaccination, helping to accelerate the global vaccination campaign and combat the spread of the virus.[2] | United States |
| 2021 | Cancer | Therapeutic development | Miao et al. report that mRNA encoding antibodies targeting tumors is an area of active research. Monoclonal antibodies (mAbs) have the ability to directly kill cancer cells or stimulate immune cells to combat cancer. Bispecific antibodies, which can bind to both T cells and cancer cells, are particularly noteworthy as they facilitate the interaction between T cells and cancer cells, leading to the destruction of cancer cells by T cells. At this time, clinical trials currently evaluate mRNA-based vaccines for various types of cancer, including colorectal cancer, prostate cancer, triple-negative breast cancer, bladder cancer, pancreatic cancer, esophageal squamous carcinoma, gastric adenocarcinoma, melanoma, and non-small cell lung carcinoma.[5][79] | |
| 2022 (November) | Multiple | Publication | Sarfaraz K. Niazi publishes mRNA Therapeutics: Fast-to-Market Strategies, a 326-page guide covering nucleic acid therapeutics, focusing on mRNA vaccines. The book details manufacturing, formulations, safety testing, and regulatory steps, emphasizing cGMP compliance. It introduces the concept of "biosimilar" mRNA vaccines for rapid approval, providing financial projections for RNA manufacturing facilities and insights into supply chain management. The text also explores the future of nucleic acid products, suggesting a shift towards nucleic acid vaccines for autoimmune disorders' prevention. The book anticipates increased industry engagement, promising significant advancements in drug development.[80] | |
| 2022 (December 29) | Melanoma | Therapeutic development | A study on melanoma treatment unveils promising results with personalized mRNA vaccines combined with the immunotherapy drug pembrolizumab. In the KEYNOTE/mRNA trial, the combination therapy significantly reduces the risk of recurrence or death by 44% compared to pembrolizumab alone in stage III/IV melanoma patients post-surgery. Although there were slightly more adverse events with the combined therapy, experts hail this advancement as a significant step in melanoma treatment. Personalized mRNA vaccines train the immune system to recognize cancer proteins, potentially revolutionizing cancer therapy. This development, reminiscent of the penicillin moment, indicates a promising future for mRNA vaccines in treating various cancers.[81] | |
| 2023 (October 2) | COVID-19 | Recognition | The Nobel Prize in Physiology or Medicine is jointly awarded to Katalin Karikó and Drew Weissman for their discoveries in nucleoside base modifications, which paved the way for the development of highly effective mRNA vaccines against COVID-19. Their research revolutionized our understanding of mRNA interaction with the immune system, facilitating the rapid development of vaccines during the pandemic. Before the pandemic, vaccines relied on whole viruses, viral components, or vector systems, requiring large-scale cell culture. Karikó and Weissman's work on mRNA technology overcame previous obstacles, offering a promising avenue for vaccine development independent of cell culture. Their breakthrough, published in 2005, anticipated the mRNA vaccine revolution witnessed during the COVID-19 pandemic.[82] | |
| 2024 (April 7) | Pancreatic cancer | Therapeutic development | In a phase 1 clinical trial, the investigational mRNA-based cancer vaccine autogene cevumeran shows promising results in stimulating a lasting immune response against pancreatic cancer. Administered alongside atezolizumab and mFOLFIRINOX chemotherapy, the vaccine activates T cells specific to neoantigens found in individual patients' tumors, potentially reducing the risk of cancer recurrence. Notably, vaccine-induced T cells persisted for up to three years after treatment in some patients. These findings support the development of personalized mRNA vaccines as a viable approach to targeting pancreatic cancer and warrant further investigation in larger clinical trials.[83] |
Numerical and visual data
Google trends
The chart below shows Google Trends data for Messenger RNA, from January 2004 to September 2023, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[84]
Google ngram viewer
The chart below shows Google Ngram Viewer data for Messenger RNA, between 1950 and 2019.[85]
Wikipedia views
The chart below shows pageviews of the English Wikipedia article Messenger RNA, from July 2015 to August 2023. See spike of interest around late 2020 during the COVID-19 pandemic, at the time of massive deployment of mRNA vaccines.[86]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by User:Sebastian.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
- Summary by decade
- https://www.science.org/content/article/mrna-discovery-paved-way-covid-19-vaccines-wins-nobel-prize-physiology-medicine multiple events
- https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/ multiple events
- https://the-dna-universe.com/2021/04/15/the-history-of-mrna-applications/ ref name="The DNA Unive"> multiple events
- https://www.pfizer.com/science/innovation/mrna-technology by decade
- https://www.theguardian.com/society/2024/apr/26/cancer-mrna-vaccine-melanoma-trial
- https://www.mskcc.org/news/can-mrna-vaccines-fight-pancreatic-cancer-msk-clinical-researchers-are-trying-find-out
- https://www.biospace.com/article/releases/mrna-therapeutics-market-size-set-to-soar-usd-58-90-bn-by-2033/
- https://theconversation.com/brain-cancer-in-children-is-notoriously-hard-to-treat-a-new-mrna-cancer-vaccine-triggers-an-attack-from-within-228666
- [1]
- [2]
- Books
- [3]
- [4] ref name [55]
- Messenger RNA
- mRNA vaccine
- mRNA display
- Missense mRNA
- Transcriptome
- [5]
Timeline update strategy
See also
External links
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Rhoads, Robert E. (2016). Synthetic MRNA: Production, Introduction Into Cells, and Physiological Consequences. Springer New York. ISBN 978-1-4939-3625-0.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 2.29 2.30 "mRNA is central to production of proteins in the body which makes it an ideal tool for therapeutics and vaccines". WhatisBiotechnology.org. Retrieved 18 March 2022.
- ↑ "The Long History of mRNA Vaccines | Johns Hopkins | Bloomberg School of Public Health". publichealth.jhu.edu. 6 October 2021. Retrieved 8 July 2023.
- ↑ "The mRNA revolution: How COVID-19 hit fast-forward on an experimental technology". New Atlas. 23 April 2021. Retrieved 2 March 2022.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 5.28 5.29 5.30 October 29th, reas Ebertz 15 April 2021 (15 April 2021). "The history of mRNA applications". The DNA Universe BLOG. Retrieved 12 March 2022.
- ↑ Kowalska, J; Wypijewska del Nogal, A; Darzynkiewicz, ZM; Buck, J; Nicola, C; Kuhn, AN; Lukaszewicz, M; Zuberek, J; Strenkowska, M; Ziemniak, M; Maciejczyk, M; Bojarska, E; Rhoads, RE; Darzynkiewicz, E; Sahin, U; Jemielity, J (2014). "Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: versatile tools for manipulation of therapeutically relevant cap-dependent processes.". Nucleic acids research. 42 (16): 10245–64. PMID 25150148. doi:10.1093/nar/gku757.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 Cobb, Matthew (June 2015). "Who discovered messenger RNA?". Current Biology. 25 (13): R526–R532. doi:10.1016/j.cub.2015.05.032.
- ↑ Avery, O. T.; Macleod, C. M.; McCarty, M. (1 February 1944). "STUDIES ON THE CHEMICAL NATURE OF THE SUBSTANCE INDUCING TRANSFORMATION OF PNEUMOCOCCAL TYPES : INDUCTION OF TRANSFORMATION BY A DESOXYRIBONUCLEIC ACID FRACTION ISOLATED FROM PNEUMOCOCCUS TYPE III". The Journal of Experimental Medicine. 79 (2): 137–158. ISSN 0022-1007. doi:10.1084/jem.79.2.137.
- ↑ Cobb, Matthew (January 2014). "Oswald Avery, DNA, and the transformation of biology". Current Biology. 24 (2): R55–R60. doi:10.1016/j.cub.2013.11.060.
- ↑ Cobb, Matthew (January 2014). "Oswald Avery, DNA, and the transformation of biology". Current Biology. 24 (2): R55–R60. doi:10.1016/j.cub.2013.11.060.
- ↑ 11.0 11.1 11.2 Oeffinger, Marlene; Zenklusen, Daniel (6 December 2019). The Biology of mRNA: Structure and Function. Springer Nature. ISBN 978-3-030-31434-7.
- ↑ Jeener, R.; Szafarz, D. (1 March 1950). "Relations Between the Rate of Renewal and the Intracellular Localization of Ribonucleic Acid". Arch. Biochem. Vol: 26.
- ↑ DOUNCE, AL (1 September 1952). "[Duplicating mechanism for peptide chain and nucleic acid synthesis].". Enzymologia. 15 (5): 251–8. PMID 13033864.
- ↑ Monod, J; Pappenheimer, A.M; Cohen-Bazire, G (January 1952). "La cinétique de la biosynthèse de la β-galactosidase chez E. coli considérée comme fonction de la croissance". Biochimica et Biophysica Acta. 9: 648–660. doi:10.1016/0006-3002(52)90227-8.
- ↑ Pardee, Arthur B. (May 1954). "NUCLEIC ACID PRECURSORS AND PROTEIN SYNTHESIS". Proceedings of the National Academy of Sciences. 40 (5): 263–270. doi:10.1073/pnas.40.5.263.
- ↑ Hershey, A. D.; Dixon, June; Chase, Martha (20 July 1953). "NUCLEIC ACID ECONOMY IN BACTERIA INFECTED WITH BACTERIOPHAGE T2". Journal of General Physiology. 36 (6): 777–789. doi:10.1085/jgp.36.6.777.
- ↑ Pardee, Arthur B. (November 2002). "PaJaMas in Paris". Trends in Genetics. 18 (11): 585–587. doi:10.1016/S0168-9525(02)02780-4.
- ↑ "Some historical remarks on Jacob-Monod operon model". hal.science. Retrieved 2 June 2023.
- ↑ Astrachan, L.; Volkin, E. (September 1958). "Properties of ribonucleic acid turnover in T2-infected Escherichia coli". Biochimica et Biophysica Acta. 29 (3): 536–544. doi:10.1016/0006-3002(58)90010-6.
- ↑ T.H. Jukes Federation meetings Nature, 267 (1977), p. 8
- ↑ Nomura, Masayasu; Hall, Benjamin D.; Spiegelman, S. (November 1960). "Characterization of RNA synthesized in Escherichia coli after bacteriophage T2 infection". Journal of Molecular Biology. 2 (5): 306–IN4. doi:10.1016/S0022-2836(60)80027-7.
- ↑ Hall, Benjamin D.; Spiegelman, S. (February 1961). "SEQUENCE COMPLEMENTARITY OF T2-DNA AND T2-SPECIFIC RNA". Proceedings of the National Academy of Sciences. 47 (2): 137–146. doi:10.1073/pnas.47.2.137.
- ↑ Fisher, Susie (October 2015). "Not just "a clever way to detect whether DNA really made RNA"1: The invention of DNA–RNA hybridization and its outcome1Judson (1979, p. 440).". Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. 53: 40–52. doi:10.1016/j.shpsc.2015.07.002.
- ↑ Aronson, Arthur I.; McCarthy, Brian J. (January 1961). "Studies of E. coli Ribosomal RNA and Its Degradation Products". Biophysical Journal. 1 (3): 215–226. doi:10.1016/S0006-3495(61)86885-9.
- ↑ Matthaei, Heinrich; Nirenberg, Marshall W. (April 1961). "The dependence of cell-free protein synthesis in E.coli upon RNA prepared from ribosomes". Biochemical and Biophysical Research Communications. 4 (6): 404–408. doi:10.1016/0006-291x(61)90298-4.
- ↑ Brenner, S.; Jacob, F.; Meselson, M. (May 1961). "An Unstable Intermediate Carrying Information from Genes to Ribosomes for Protein Synthesis". Nature. 190 (4776): 576–581. doi:10.1038/190576a0.
- ↑ Gros, Francois; Hiatt, H.; Gilbert, Walter; Kurland, C. G.; Risebrough, R. W.; Watson, J. D. (May 1961). "Unstable Ribonucleic Acid Revealed by Pulse Labelling of Escherichia Coli". Nature. 190 (4776): 581–585. doi:10.1038/190581a0.
- ↑ ECK, RV (23 September 1961). "Non-randomness in amino-acid 'alleles'.". Nature. 191: 1284–5. PMID 13889081. doi:10.1038/1911284a0.
- ↑ Charles Yanofsky. "Establishing the Triplet Nature of the Genetic Code" (PDF). Cell. doi:10.1016/j.cell.2007.02.029. Retrieved 16 September 2024.
- ↑ "Nirenberg Introduction". history.nih.gov. Retrieved 21 September 2024.
- ↑ "The Experiment that Cracked the Code of Life". oligofastx.com. Retrieved 21 September 2024.
- ↑ "Cracking the Genetic Code: Replicating a Scientific Discovery". scienceinschool.org. Retrieved 21 September 2024.
- ↑ Isaacs, Alick (1964). "Interferon". Advances in Virus Research. 10: 1–38. doi:10.1016/S0065-3527(08)60695-8.
- ↑ "The Genetic Code: Deciphering the Blueprint of Life". American Chemical Society. Retrieved 16 September 2024.
- ↑ Chapoval, Svetlana P. (7 November 2018). "Dendritic Cells". intechopen. Retrieved 16 May 2022.
- ↑ Furuichi, Yasuhiro; Shatkin, Aaron J (2000). "Viral and cellular mRNA capping: Past and prospects". Advances in Virus Research. 55: 135–184. doi:10.1016/s0065-3527(00)55003-9.
- ↑ "The Nobel Prize in Physiology or Medicine 1993". NobelPrize.org. Retrieved 3 August 2024.
- ↑ "MIT Professor Phillip Sharp wins Nobel Prize in medicine". MIT News. Retrieved 3 August 2024.
- ↑ "The discovery of split genes and RNA splicing". ResearchGate. Retrieved 3 August 2024.
- ↑ "Discoveries of split genes". AnimalResearch.info. Retrieved 3 August 2024.
- ↑ Dimitriadis, Giorgos J. (August 1978). "Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes". Nature. 274 (5674): 923–924. ISSN 1476-4687. doi:10.1038/274923a0.
- ↑ Ostro, M. J.; Giacomoni, D.; Lavelle, D.; Paxton, W.; Dray, S. (31 August 1978). "Evidence for translation of rabbit globin mRNA after liposome-mediated insertion into a human cell line". Nature. 274 (5674): 921–923. ISSN 0028-0836. doi:10.1038/274921a0.
- ↑ "The Nobel Prize in Chemistry 1989". Nobel Prize. Retrieved 3 September 2024.
- ↑ Dolgin, Elie (14 September 2021). "The tangled history of mRNA vaccines". Nature. pp. 318–324. doi:10.1038/d41586-021-02483-w. Retrieved 16 May 2022.
- ↑ Tabor, S; Richardson, C C (February 1985). "A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes.". Proceedings of the National Academy of Sciences. 82 (4): 1074–1078. doi:10.1073/pnas.82.4.1074.
- ↑ MD, Justus R. Hope. "Inventor of mRNA banned by the New England Journal of Medicine". The Desert Review. Retrieved 5 March 2022.
- ↑ Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ (November 2012). "Developing mRNA-vaccine technologies". RNA Biology. 9 (11): 1319–30. PMC 3597572
 . PMID 23064118. doi:10.4161/rna.22269.
. PMID 23064118. doi:10.4161/rna.22269.
- ↑ Wolff, Jon A.; Malone, Robert W.; Williams, Phillip; Chong, Wang; Acsadi, Gyula; Jani, Agnes; Felgner, Philip L. (23 March 1990). "Direct Gene Transfer into Mouse Muscle in Vivo". Science. 247 (4949): 1465–1468. doi:10.1126/science.1690918.
- ↑ 49.0 49.1 Pardi, Norbert; Hogan, Michael J.; Porter, Frederick W.; Weissman, Drew (April 2018). "mRNA vaccines — a new era in vaccinology". Nature Reviews Drug Discovery. pp. 261–279. doi:10.1038/nrd.2017.243. Retrieved 5 March 2022.
- ↑ Wolff, Jon A.; Malone, Robert W.; Williams, Phillip; Chong, Wang; Acsadi, Gyula; Jani, Agnes; Felgner, Philip L. (23 March 1990). "Direct Gene Transfer into Mouse Muscle in Vivo". Science. 247 (4949): 1465–1468. doi:10.1126/science.1690918.
- ↑ Dolgin, Elie (14 September 2021). "The tangled history of mRNA vaccines". Nature. pp. 318–324. doi:10.1038/d41586-021-02483-w. Retrieved 9 March 2022.
- ↑ Jirikowski, Gustav F.; Sanna, Pietro Paolo; Maciejewski-Lenoir, Dominique; Bloom, Floyd E. (21 February 1992). "Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA". Science. 255 (5047): 996–998. doi:10.1126/science.1546298.
- ↑ Jirikowski, Gustav F.; Sanna, Pietro Paolo; Maciejewski-Lenoir, Dominique; Bloom, Floyd E. (21 February 1992). "Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA". Science. 255 (5047): 996–998. doi:10.1126/science.1546298.
- ↑ Belasco, Joel G.; Brawerman, George (20 April 1993). Control of Messenger RNA Stability. Elsevier Science. ISBN 978-0-12-084782-2.
- ↑ 55.0 55.1 Babendure, Jeremy R.; Babendure, Jennie L.; Ding, Jian-Hua; Tsien, Roger Y. "Control of mammalian translation by mRNA structure near caps". RNA. 12 (5): 851–861. ISSN 1355-8382. doi:10.1261/rna.2309906.
- ↑ Conry, R. M.; LoBuglio, A. F.; Wright, M.; Sumerel, L.; Pike, M. J.; Johanning, F.; Benjamin, R.; Lu, D.; Curiel, D. T. (1 April 1995). "Characterization of a messenger RNA polynucleotide vaccine vector". Cancer Research. 55 (7): 1397–1400. ISSN 0008-5472.
- ↑ MacLachlan, Ian; Cullis, Pieter (2005). ""Diffusible‐PEG‐Lipid Stabilized Plasmid Lipid Particles"". Advances in Genetics. 53: 157–188. doi:10.1016/S0065-2660(05)53006-2.
- ↑ Roberts RW, Szostak JW (1997). "RNA-peptide fusions for the in vitro selection of peptides and proteins". Proc Natl Acad Sci USA. 94 (23): 12297–302. PMC 24913
 . PMID 9356443. doi:10.1073/pnas.94.23.12297
. PMID 9356443. doi:10.1073/pnas.94.23.12297 .
.
- ↑ 59.00 59.01 59.02 59.03 59.04 59.05 59.06 59.07 59.08 59.09 59.10 59.11 59.12 59.13 59.14 "Heavy! Global mRNA Pharmaceutical Panorama.". topic.echemi.com. Retrieved 21 April 2022.
- ↑ "Merix Bioscience, Inc". The Case Centre. Retrieved 9 June 2022.
- ↑ Richter, Joel D.; Richter, Professor in the Department of Molecular Genetics and Microbiology Joel D. (16 October 1997). MRNA Formation and Function. Elsevier Science. ISBN 978-0-12-587545-5.
- ↑ Karikó, Katalin; Buckstein, Michael; Ni, Houping; Weissman, Drew (August 2005). "Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA". Immunity. 23 (2): 165–175. doi:10.1016/j.immuni.2005.06.008.
- ↑ Kramps, Thomas; Elbers, Knut (2017). RNA Vaccines: Methods and Protocols. Springer New York. ISBN 978-1-4939-6481-9.
- ↑ Tratschin, Jon Duri; Ruggli, Nicolas; McCullough, Kenneth Charles (10 December 2009). "Pestivirus replicons providing an rna-based viral vector system". Retrieved 5 March 2022.
- ↑ Warren, Luigi; Manos, Philip D.; Ahfeldt, Tim; Loh, Yuin-Han; Li, Hu; Lau, Frank; Ebina, Wataru; Mandal, Pankaj K.; Smith, Zachary D.; Meissner, Alexander; Daley, George Q.; Brack, Andrew S.; Collins, James J.; Cowan, Chad; Schlaeger, Thorsten M.; Rossi, Derrick J. (5 November 2010). "Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA". Cell Stem Cell. 7 (5): 618–630. ISSN 1875-9777. doi:10.1016/j.stem.2010.08.012.
- ↑ Yakubov, Eduard; Rechavi, Gidi; Rozenblatt, Shmuel; Givol, David (26 March 2010). "Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors". Biochemical and Biophysical Research Communications. 394 (1): 189–193. ISSN 1090-2104. doi:10.1016/j.bbrc.2010.02.150.
- ↑ Jinek, Martin; Chylinski, Krzysztof; Fonfara, Ines; Hauer, Michael; Doudna, Jennifer A.; Charpentier, Emmanuelle (17 August 2012). "A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity". Science (New York, N.Y.). 337 (6096): 816–821. ISSN 1095-9203. doi:10.1126/science.1225829.
- ↑ "AstraZeneca and Moderna Therapeutics Announce Collaboration for Cardiometabolic Diseases and Cancer Treatment". AstraZeneca. 21 March 2013. Retrieved 16 September 2024.
- ↑ "Lilly and BioNTech Announce Research Collaboration on Novel Cancer Immunotherapies". investor.lilly.com. Retrieved 4 June 2023.
- ↑ "CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing". PLOS ONE. doi:10.1371/journal.pone.0273864. Retrieved 16 September 2024.
- ↑ Stadler, Christiane R.; Bähr-Mahmud, Hayat; Celik, Leyla; Hebich, Bernhard; Roth, Alexandra S.; Roth, René P.; Karikó, Katalin; Türeci, Özlem; Sahin, Ugur (July 2017). "Elimination of large tumors in mice by mRNA-encoded bispecific antibodies". Nature Medicine. 23 (7): 815–817. ISSN 1546-170X. doi:10.1038/nm.4356.
- ↑ "Genmab and CureVac Enter Strategic Partnership to Develop mRNA-based Antibody Therapeutics". ir.genmab.com. Retrieved 5 June 2023.
- ↑ Pardi, Norbert; Secreto, Anthony J.; Shan, Xiaochuan; Debonera, Fotini; Glover, Joshua; Yi, Yanjie; Muramatsu, Hiromi; Ni, Houping; Mui, Barbara L.; Tam, Ying K.; Shaheen, Farida; Collman, Ronald G.; Karikó, Katalin; Danet-Desnoyers, Gwenn A.; Madden, Thomas D.; Hope, Michael J.; Weissman, Drew (2 March 2017). "Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge". Nature Communications. 8: 14630. ISSN 2041-1723. doi:10.1038/ncomms14630.
- ↑ Schlake, Thomas; Thran, Moritz; Fiedler, Katja; Heidenreich, Regina; Petsch, Benjamin; Fotin-Mleczek, Mariola (10 April 2019). "mRNA: A Novel Avenue to Antibody Therapy?". Molecular Therapy: The Journal of the American Society of Gene Therapy. 27 (4): 773–784. ISSN 1525-0024. doi:10.1016/j.ymthe.2019.03.002.
- ↑ Oeffinger, Marlene; Zenklusen, Daniel (6 December 2019). The Biology of mRNA: Structure and Function. Springer Nature. ISBN 978-3-030-31434-7.
- ↑ "Pfizer and BioNTech to Co-Develop Potential COVID-19 Vaccine | Pfizer". www.pfizer.com. Retrieved 5 June 2023.
- ↑ "GSK and CureVac announce strategic mRNA technology collaboration | GSK". www.gsk.com. 20 July 2020. Retrieved 5 June 2023.
- ↑ Commissioner, Office of the (14 December 2020). "FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine". FDA. Retrieved 10 June 2022.
- ↑ Miao, Zhen; Balzer, Michael S.; Ma, Ziyuan; Liu, Hongbo; Wu, Junnan; Shrestha, Rojesh; Aranyi, Tamas; Kwan, Amy; Kondo, Ayano; Pontoglio, Marco; Kim, Junhyong; Li, Mingyao; Kaestner, Klaus H.; Susztak, Katalin (15 April 2021). "Single cell regulatory landscape of the mouse kidney highlights cellular differentiation programs and disease targets". Nature Communications. 12 (1): 2277. ISSN 2041-1723. doi:10.1038/s41467-021-22266-1.
- ↑ Niazi, Sarfaraz K. (6 December 2022). "mRNA Therapeutics: Fast-to-Market Strategies". books.google.com.ar. CRC Press. Retrieved 3 October 2023.
- ↑ "Breakthrough in mRNA vaccines for melanoma". racgp.org.au. Retrieved 27 May 2024.
- ↑ "The Nobel Prize in Physiology or Medicine 2023". NobelPrize.org. Retrieved 27 May 2024.
- ↑ Stallard, Jim (7 April 2024). "Investigational mRNA Vaccine Induced Persistent Immune Response in Phase 1 Trial of Patients With Pancreatic Cancer | Memorial Sloan Kettering Cancer Center". www.mskcc.org. Retrieved 28 May 2024.
- ↑ "Messenger RNA". trends.google.com. Retrieved 12 September 2023.
- ↑ "Messenger RNA". books.google.com. Retrieved 13 September 2023.
- ↑ "Messenger RNA". wikipediaviews.org. Retrieved 13 September 2023.