Timeline of lithium
This is a timeline of lithium, attempting to focus both on the scientific development involving the element and on its industrial development.
Big picture
| Time period | Development summary |
|---|---|
| 19th century | Lithium is discovered and first isolated; and large quantities of the metal are already obtained by electrolysis. In the last half of the century, lithium salts and mineral water containing them are used to treat gout (later proved unsuccessful) and to ward off depression.[1] Lithium is also used to treat epilepsy and cancer.[2] |
| 20th century | The use of lithium falls into medical disrepute early in the century.[1] However, since the end of World War 2 and during the Cold War, the demand for lithium and its production increases dramatically with the production of nuclear fusion weapons. By the 1950s, the United States government is the largest consumer of lithium, and the country would remain the prime producer of lithium until the mid 1980s. In the early 1970s the first non-rechargeable lithium batteries become commercially available. Attempts to develop rechargeable lithium batteries follow in the 1980s but fail because of instabilities in the metallic lithium used as anode material.[3] The first commercially-viable rechargeable lithium ion battery are produced in the 1990s.[4] the rechargeable lithium battery sector soon becomes a highly competitive, low-margin industry dominated by a few firms based mainly in Japan.[5] |
| 21th century | The development of lithium ion batteries increases the demand for lithium and becomes the dominant use.[6] While historically the lithium market was dominated by American production from mineral deposits, by the 21th century Australia, Chile, and Portugal became the world’s largest suppliers of the metal.[1] World production is currently on the order of 2 × 10^7 kg per year.[7] As of 2017, over 61 percent of the world’s lithium reserves are contained within the “Lithium Triangle”, a region overlaying Argentina, Bolivia and Chile.[8] |
Full timeline
| Year | Event type | Details |
|---|---|---|
| 1817 | Discovery | Swedish chemist Johan August Arfvedson discovers the chemical element lithium in Stockholm, Sweden, during an analysis of petalite ore ((LiAl(Si2O5)2) ).[9][10] Arfwedson calls it lithium, from the Greek word for stone.[4] |
| 1818 | Scientific development | German chemist Christian Gmelin observes that lithium salts colour flames bright red.[9] |
| 1855 | Scientific development | Thomas Brande isolates lithium as a free metal.[4] |
| 1855 | Scientific development | German chemist Robert Bunsen and British chemist Augustus Mattiessen manage to isolate large quantities of lithium by electrolysis of lithium chloride.[9] |
| 1870s | Application (medicine) | Carl Lange in Denmark[11] and William Alexander Hammond in New York City[12] start using lithium to treat mania. |
| 1912 | Application (energy) | American chemist Gilbert N. Lewis begins exploring the possibility of lithium batteries.[4] |
| 1923 | Production | German industrial conglomerate Metallgesellschaft produces the first commercial quantities of lithium metal.[13][14] |
| 1930–1936 | Application (food) | Grigg starts producing a “lithiated” drink called Bib-Label Lithiated Lemon-Line Soda. The promotion of the product centers the healthful aspects of the drink, stemmed from lithium’s benefit of evening out moods swings. Welcomed by the public, the drink adopts its final name 7Up, the name it is known by today.[4] |
| 1932 | Scientific development | British physicist John Cockcroft and Irish physicist Ernest Walton use lithium as the target metal in the pioneering work of transmuting nuclei by artificially accelerated atomic particles. Cockroft and Walton would produce a nuclear disintegration by bombarding Lithium with artificially accelerated protons. Each lithium nucleus that absorbed a proton would become two helium nuclei. The bombardment of lithium-6 with slow neutrons produces helium and tritium (3H); this reaction is a major source of tritium production.[1][15] |
| 1942 | Organization | Lithium Corporation of America is founded by the United States government for production of Lithium-7 in the Manhattan Project for the development of the hydrogen bomb.[4] |
| 1948 | Application (medicine) | Australian psychiatrist John Cade starts using lithium in the treatment of mental disorder.[2] |
| 1953 | Application (edicine) | The United States Atomic Energy Commission (AEC) requires large amounts of lithium hydroxide for use in the production of thermonuclear weapons.[4] |
| 1954 (March 1) | Application (military) | Castle Bravo nuclear test is conducted. Using solid lithium deuteride, it produces a yield of 15 megatons, 1,000 times as large as the Hiroshima bomb.[16] |
| 1954 | Application (medicine) | The use of lithium carbonate to treat manic-depression (also known as bipolar disorder) is demonstrated clinically.[1] |
| 1966 | Application (medicine) | Ronald R. Fieve opens the first lithium clinic in North America.[17] |
| 1967 | Application (medicine) | Lithium carbonate is introduced as a drug.[18] |
| 1970 | Application (medicine) | The application of lithium in manic illness is approved by the United States Food and Drug Administration.[19] |
| 1973 | Application (energy | Israeli-American engineer Adam Heller proposes the lithium thionyl chloride battery.[20] |
| 1975 | Consumption | Estimated world consumption of lithium is 4090 tonnes in the year.[21] |
| 1975–1980 | Application (energy) | French chemist Michel Armand first formulates the idea of a battery in which the lithium ion moves reversibly between the positive and negative electrodes.[22] Armand establishes the electrical properties of the polymer-salt complex formed with lithium and pointes out that this material would be useful for batteries. |
| 1977 | Application (energy) | Samar Basu, at the University of Pennsylvania, demonstrates electrochemical intercalation of lithium in graphite.[23][24] This would lead to the development of a workable lithium intercalated graphite electrode at Bell Labs LiC6 to provide an alternative to the lithium metal electrode battery. |
| 1979 | Application (energy) | Ned A. Godshall et al. at Stanford University demonstrated a rechargeable lithium cell with voltage in the 4 V range using lithium cobalt oxide (LiCoO2) as the positive electrode and lithium metal as the negative electrode.[25][26] This innovation provides the positive electrode material that would make lithium batteries commercially possible.[27][28][29] |
| 1980 | Application (energy) | French Moroccan scientist Rachid Yazami demonstrates the reversible electrochemical intercalation of lithium in graphite.[30][31] Yazami is the inventor of the graphite anode (negative pole) of lithium ion batteries.[32] |
| 1980 | Application (energy) | American physicist John B. Goodenough invents a new type of lithium battery in which the lithium (Li) could migrate through the battery from one electrode to the other as a Li+ ion.[33] |
| 1991 | Application (energy) | Sony Corporation announces a new product called a lithium-ion battery.[22] |
| 1990s | Application (energy) | John B. Goodenough introduces a stable lithium-ion cathode based on lithium iron and phosphate.[33] |
| 1990s | Application (energy) | Sony manufactures lithium cobalt oxide (LiCoO2) batteries, which would often catch fire.[33] |
| 1991 | Application (energy) | Sony announce an international patent on a lithium-cobalt-oxide cathode.[3] |
| 2008 | Application (medicine) | Researchers report in the journal Cell that lithium interrupts the activity of a receptor for the neurotransmitter dopamine.[34] |
| 2010 (January) | Reserves | The United States Geological Survey estimates world total lithium reserves at 9.9×109 kg (economically extractable) and identifies lithium resources at 2.55 × 1010 kg (potentially economic). Most of the identified resources are in Bolivia and Chile, with 9 × 109 kg and 7.5 × 109 kg, respectively.[7] |
| 2011 | Research | Study at the University of Michigan and Ford Motor Company finds enough resources to support global lithium demand until 2100, including the lithium required for the potential widespread transportation use.[35] |
| 2012 | Production | California-based Simbol Materials develops a technology to separate lithium from brine produced by geothermal power plants.[36] |
| 2013 | Production | Korean multinational steel company Posco develops a method to extract lithium from continental brine.[37] |
| 2016 | Policy | After lithium batteries having burned in commercial jet aircrafts, the International Civil Aviation Organization bans the shipment of lithium ion batteries as cargo on passenger aircraft.[5] |
| 2016 | Consumption | The global demand of metalic lithium is of 37,800 tonnes.[38] |
| 2018 | Reserves | Worldwide identified lithium reserves are estimated by the United States Geological Survey (USGS) to be 16 million tonnes.[39] |
| 2018 (May) | Application (energy) | Researchers at Yale and Donghua University in China develop a new way to create lithium metal electrodes for batteries.[40] |
| 2021 (June) | Research | Researchers at Saudi Arabia's King Abdullah University of Science and Technology experimentally validates a device that can cheaply extract lithium from seawater, while desalinating it and producing more than enough hydrogen and chlorine gases to pay its power bills.[41] |
Numerical and visual data
Google Scholar
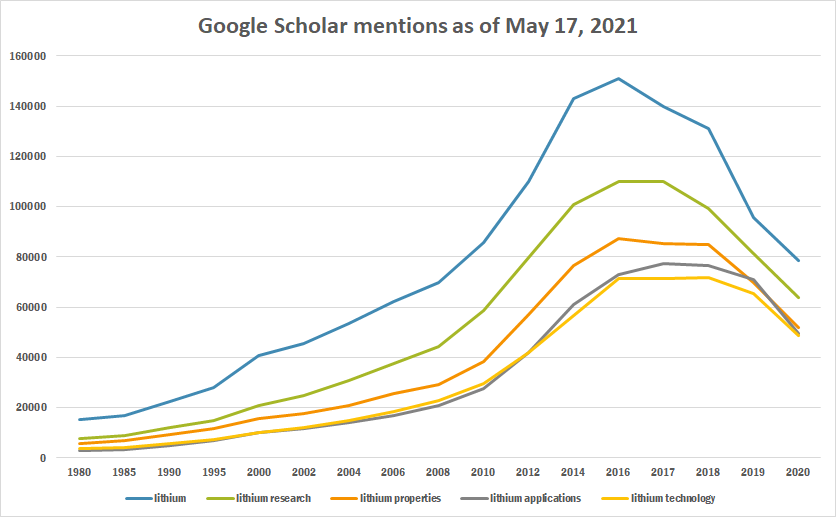
The table below summarizes per-year mentions on Google Scholar as of May 17, 2021.
| Year | lithium | lithium research | lithium properties | lithium applications | lithium technology |
|---|---|---|---|---|---|
| 1980 | 15,200 | 7,590 | 5,810 | 2,770 | 3,550 |
| 1985 | 16,700 | 9,020 | 6,770 | 3,250 | 3,960 |
| 1990 | 22,200 | 12,200 | 9,370 | 4,970 | 5,550 |
| 1995 | 28,100 | 14,800 | 11,700 | 6,780 | 7,280 |
| 2000 | 40,900 | 20,800 | 15,700 | 9,920 | 10,200 |
| 2002 | 45,500 | 24,700 | 17,700 | 11,600 | 11,900 |
| 2004 | 53,300 | 30,600 | 20,800 | 14,100 | 15,000 |
| 2006 | 62,200 | 37,700 | 25,400 | 16,900 | 18,500 |
| 2008 | 69,600 | 44,400 | 29,200 | 20,600 | 22,600 |
| 2010 | 85,900 | 58,700 | 38,400 | 27,600 | 29,700 |
| 2012 | 110,000 | 79,600 | 57,200 | 41,800 | 42,000 |
| 2014 | 143,000 | 101,000 | 76,700 | 61,100 | 56,700 |
| 2016 | 151,000 | 110,000 | 87,200 | 73,100 | 71,300 |
| 2017 | 140,000 | 110,000 | 85,200 | 77,200 | 71,200 |
| 2018 | 131,000 | 99,400 | 85,100 | 76,600 | 71,600 |
| 2019 | 95,500 | 81,300 | 69,700 | 71,100 | 65,400 |
| 2020 | 78,500 | 64,000 | 51,900 | 49,400 | 48,500 |
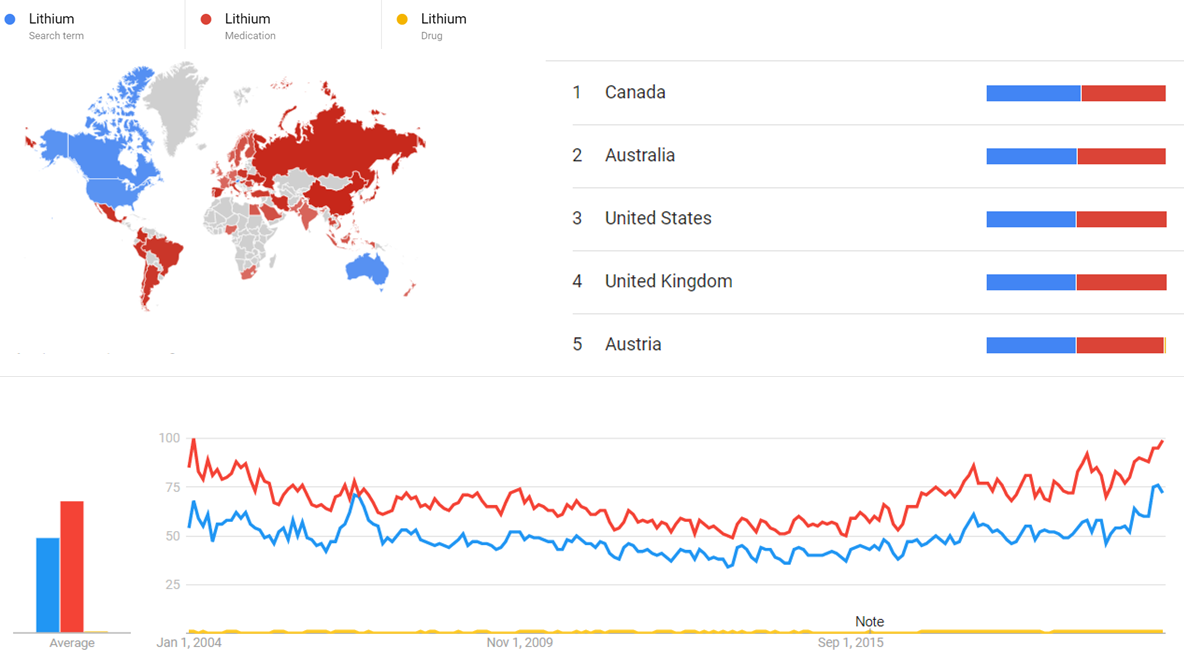
Google Trends
The comparative chart below shows Google Trends data for Lithium (Search term), Lithium (Medication) and Lithium (Drug), from January 2004 to March 2021, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[42]
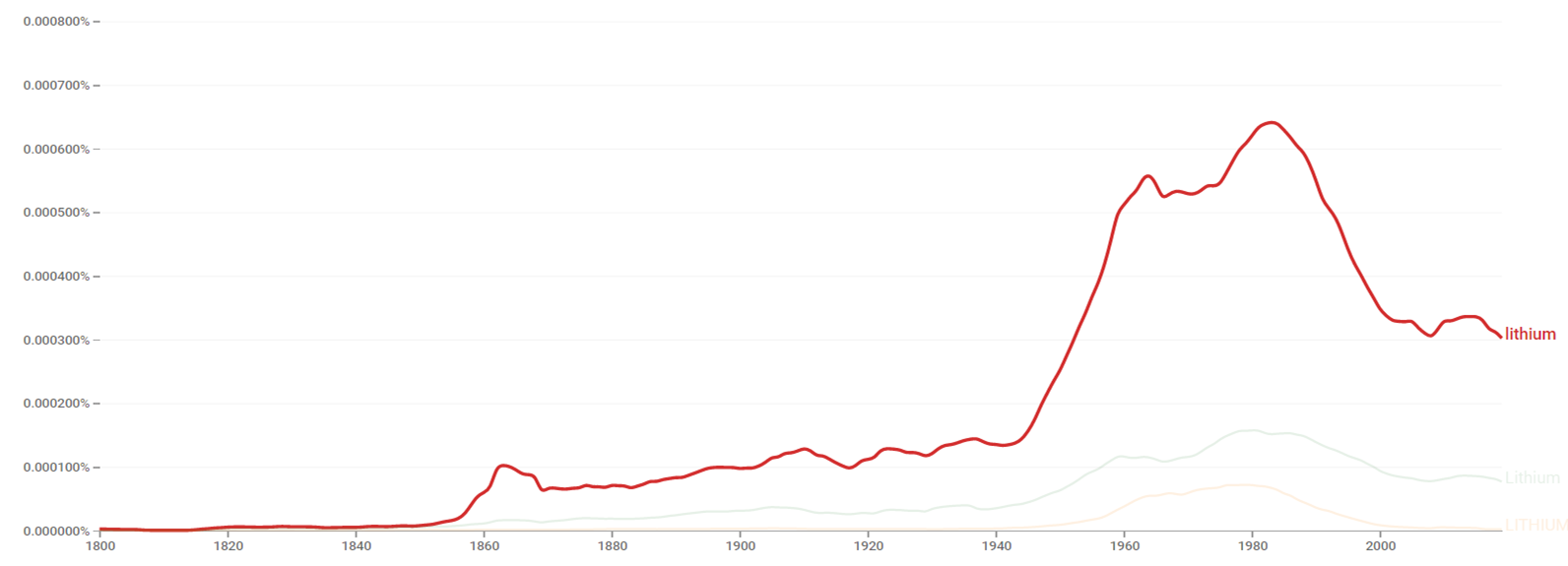
Google Ngram Viewer
The chart below shows Google Ngram Viewer data for Lithium, from 1800 to 2019.[43]
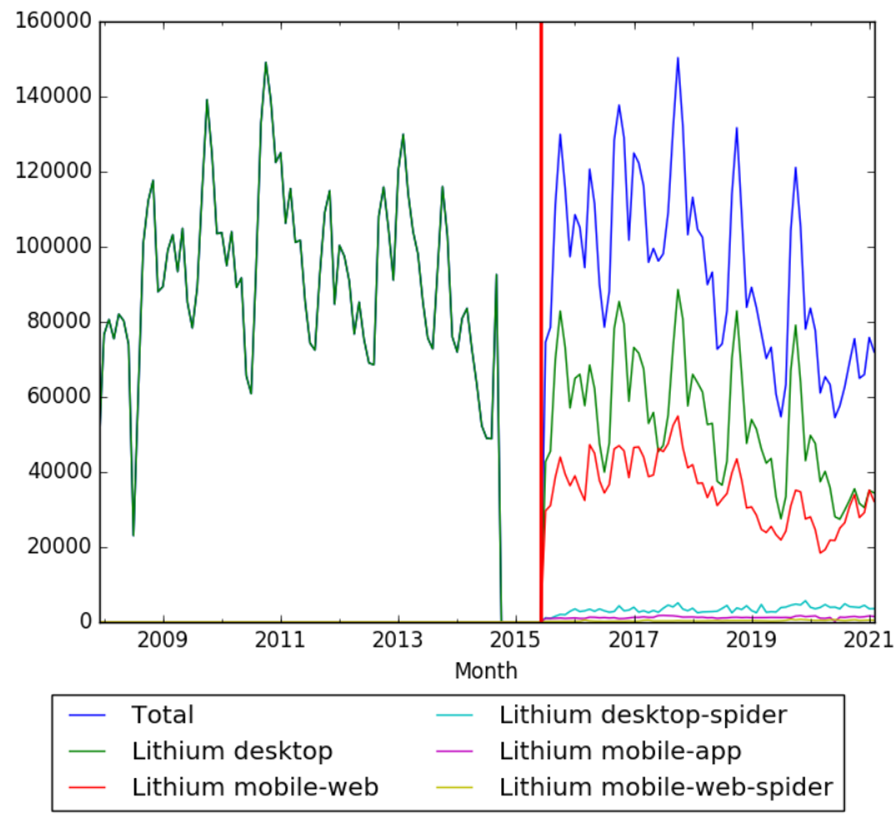
Wikipedia Views
The chart below shows pageviews of the English Wikipedia article Lithium, on desktop from December 2007, and on mobile-web, desktop-spider, mobile-web-spider and mobile app, from July 2015; to February 2021.[44]
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by User:Sebastian.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
Timeline update strategy
See also
External links
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Lithium CHEMICAL ELEMENT". britannica.com. Retrieved 16 May 2018.
- ↑ 2.0 2.1 Sneader, Walter (2005). Drug discovery : a history (Rev. and updated ed.). Chichester: Wiley. p. 63. ISBN 9780471899792. Archived from the original on 2017-09-08.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ↑ 3.0 3.1 "BU-204: How do Lithium Batteries Work?". batteryuniversity.com. Retrieved 26 May 2018.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "Lithium History". fmclithium.com. Retrieved 15 May 2018.
- ↑ 5.0 5.1 "The History of Lithium Ion Batteries Is Explosive". slate.com. Retrieved 16 May 2018.
- ↑ Ober, Joyce A. (1994). "Commodity Report 1994: Lithium" (PDF). United States Geological Survey. Archived from the original (PDF) on 9 June 2010. Retrieved 3 November 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ↑ 7.0 7.1 "World Lithium Supply". large.stanford.edu. Retrieved 16 May 2018.
- ↑ "Argentina Lithium progressing in the Lithium Triangle". investorintel.com. Retrieved 16 May 2018.
- ↑ 9.0 9.1 9.2 "Lithium: historical information". webelements.com. Retrieved 15 May 2018.
- ↑ "The Element Lithium". education.jlab.org. Retrieved 15 May 2018.
- ↑ Lenox, RH; Watson, DG (1994). "Lithium and the brain: a psychopharmacological strategy to a molecular basis for manic depressive illness". Clinical Chemistry. 40 (2): 309–14. PMID 8313612.
- ↑ Mitchell, PB; Hadzi-Pavlovic, D (2000). "Lithium treatment for bipolar disorder" (PDF). Bulletin of the World Health Organization. 78 (4): 515–7. PMC 2560742. PMID 10885179. Archived from the original (PDF) on 2012-04-01.
{{cite journal}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ↑ Information Circular, Issues 8045-8059. U.S. Department of the Interior, Bureau of Mines, 1961.
- ↑ "Lithium - Discovery, Occurrence, Properties, Production and Applications of Lithium". azom.com. Retrieved 15 May 2018.
- ↑ "Cockroft and Walton Experiment Converting Mass into Energy". homepage.eircom.net. Retrieved 16 May 2018.
- ↑ "The First Hydrogen Bombs". britannica.com. Retrieved 16 May 2018.
- ↑ "Ronald Fieve, 87; pioneered lithium to treat mood swings". bostonglobe.com. Retrieved 16 May 2018.
- ↑ Hamilton, Ian. Robert Lowell: A Biography.
- ↑ P. B. Mitchell, D. Hadzi-Pavlovic; Hadzi-Pavlovic (2000). "Lithium treatment for bipolar disorder" (PDF). Bulletin of the World Health Organization. 78 (4): 515–7. PMC 2560742. PMID 10885179. Archived from the original (PDF) on 2006-05-25.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ↑ "Electrochemical cell". patents.google.com. Retrieved 15 May 2018.
- ↑ The Australian Mineral Industry Review. Australian Government Pub. Service for the Bureau of Mineral Resources, Geology, and Geophysics., 1977.
- ↑ 22.0 22.1 Blomgren, George E. "The Development and Future of Lithium Ion Batteries". jes.ecsdl.org. Retrieved 15 May 2018.
- ↑ Zanini, M.; Basu, S.; Fischer, J. E. (1978). "Alternate synthesis and reflectivity spectrum of stage 1 lithium—graphite intercalation compound". Carbon. 16 (3): 211–212. doi:10.1016/0008-6223(78)90026-X.
- ↑ Basu, S.; Zeller, C.; Flanders, P. J.; Fuerst, C. D.; Johnson, W. D.; Fischer, J. E. (1979). "Synthesis and properties of lithium-graphite intercalation compounds". Materials Science and Engineering. 38 (3): 275–283. doi:10.1016/0025-5416(79)90132-0.
- ↑ "USPTO search for inventions by "Goodenough, John"". Patft.uspto.gov. Retrieved 8 October 2011.
- ↑ Mizushima, K.; Jones, P. C.; Wiseman, P. J.; Goodenough, J. B. (1980). "A new cathode material for batteries of high energy density". Materials Research Bulletin. 15 (6): 783–789. doi:10.1016/0025-5408(80)90012-4.
- ↑ Godshall, N.A.; Raistrick, I.D.; Huggins, R.A. (1980). "Thermodynamic investigations of ternary lithium-transition metal-oxygen cathode materials". Materials Research Bulletin. 15 (5): 561. doi:10.1016/0025-5408(80)90135-X.
- ↑ Godshall, Ned A. (17 October 1979) "Electrochemical and Thermodynamic Investigation of Ternary Lithium -Transition Metal-Oxide Cathode Materials for Lithium Batteries: Li2MnO4 spinel, LiCoO2, and LiFeO2", Presentation at 156th Meeting of the Electrochemical Society, Los Angeles, CA.
- ↑ Godshall, Ned A. (18 May 1980) Electrochemical and Thermodynamic Investigation of Ternary Lithium-Transition Metal-Oxygen Cathode Materials for Lithium Batteries. Ph.D. Dissertation, Stanford University
- ↑ International Meeting on Lithium Batteries, Rome, 27–29 April 1982, C.L.U.P. Ed. Milan, Abstract #23
- ↑ Yazami, R.; Touzain, P. (1983). "A reversible graphite-lithium negative electrode for electrochemical generators". Journal of Power Sources. 9 (3): 365–371. doi:10.1016/0378-7753(83)87040-2.
- ↑ "Rachid Yazami". NAE Website.
- ↑ 33.0 33.1 33.2 "The history and development of batteries". phys.org. Retrieved 15 May 2018.
- ↑ "Facts About Lithium". livescience.com. Retrieved 16 May 2018.
- ↑ "University of Michigan and Ford researchers see plentiful lithium resources for electric vehicles". Green Car Congress. 3 August 2011. Archived from the original on 16 September 2011. Retrieved 16 May 2018.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ↑ Fleischfresser, Channtal. "Using salt water to power our batteries | ZDNet". ZDNet. Retrieved 16 May 2018.
- ↑ "A paradigm shift for lithium?". bnamericas.com. Retrieved 16 May 2018.
- ↑ "Litio, el "oro blanco" de la Argentina". lanacion.com.ar. Retrieved 16 May 2018.
- ↑ Lithium Statistics and Information, U.S. Geological Survey, 2018
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ↑ "Chemists find new way to create lithium metal electrodes for batteries". phys.org. Retrieved 16 May 2018.
- ↑ "New tech cheaply produces lithium and H2, while desalinating seawater". New Atlas. 2021-06-07. Retrieved 8 June 2021.
- ↑ "Lithium". Google Trends. Retrieved 9 March 2021.
- ↑ "Lithium". books.google.com. Retrieved 30 March 2021.
- ↑ "Lithium". wikipediaviews.org. Retrieved 9 March 2021.