Timeline of proteins
This is a timeline of proteins, a class of macromolecules in the form of polymer chains made of amino acids. Proteins are essential nutrients for the human body. This timeline focuses on proteins from a nutritional perspective.
Contents
Sample questions
The following are some interesting questions that can be answered by reading this timeline:
- What are some notable scientific events related to the nutritional aspects of proteins?
- Sort the full timeline by "Event type" and look for the group of rows with value "Scientific development".
- What are some notable reviews expressed by experts and competent entities?
- Sort the full timeline by "Event type" and look for the group of rows with value "Notable comment".
- You will read reviews by both known experts in the field and organizations, such as the American Dietetic Association.
- What are some notable recommendations of protein intake issued by competent entities?
- What are some notable publications specialized in proteins?
- Sort the full timeline by "Event type" and look for the group of rows with value "Literature".
- Other events are described under the following types: "Focus trend", "Notable report", "Policy", and "Program launch".
Big picture
| Time period | Development summary | More details |
|---|---|---|
| 1920–1950 | Relegated attention | Around this time, nutritional research centers on the discovery of individual vitamins and amino acids, putting little interest in human protein requirements, or in the possibility of their not being met.[1] |
| 1950s–1960s | Mainstream focus | Protein again receives priority attention, with United Nations (UN) agencies being focused on protein malnutrition as the major worldwide nutritional problem.[2] The "World Protein Gap" is considered the major cause of infant mortality and retarded development in the Third World but a problem that could be solved by the application of sophisticated technology.[1] From the 1950s to the mid-1970s, United Nations (UN) agencies were focused on protein malnutrition as the major worldwide nutritional problem. |
| 1970s | Focus decline | United Nations agencies start suspecting about a "protein gap". In the mid-decade, protein malnutrition is suddenly discarded, putting end to years of agencies and international conferences focusing on protein malnutrition.[2] |
Tabular and visual data
The table below shows daily protein intake (g) by age, education, work, status, income and geographical region, in Chinese women from 1991 to 2015.[3]
| 1991 Mean | 1991 SE | 1993 Mean | 1993 SE | 1997 Mean | 1997 SE | 2000 Mean | 2000 SE | 2004 Mean | 2004 SE | 2006 Mean | 2006 SE | 2009 Mean | 2009 SE | 2011 Mean | 2011 SE | 2015 Mean | 2015 SE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group (year): | ||||||||||||||||||
| 18-49 | 70.3 | 0.4 | 69.9 | 0.4 | 67.9 | 0.4 | 65.7 | 0.4 | 65.0 | 0.4 | 65.2 | 0.4 | 63.9 | 0.4 | 61.0 | 0.4 | 57.7 | 0.4 |
| 50-64 | 67.7 | 0.7 | 66.3 | 0.9 | 63.6 | 0.8 | 63.9 | 0.7 | 64.8 | 0.7 | 64.6 | 0.6 | 62.4 | 0.6 | 59.0 | 0.5 | 56.2 | 0.4 |
| Education level: | ||||||||||||||||||
| Primary/ illiterate | 69.6 | 0.5 | 68.9 | 0.5 | 66.0 | 0.5 | 63.7 | 0.5 | 64.1 | 0.5 | 64.5 | 0.5 | 62.7 | 0.5 | 58.0 | 0.5 | 57.0 | 0.5 |
| Middle school | 69.9 | 0.6 | 69.8 | 0.7 | 67.9 | 0.6 | 66.3 | 0.6 | 64.2 | 0.6 | 65.6 | 0.6 | 63.9 | 0.5 | 60.8 | 0.5 | 58.2 | 0.6 |
| High/above | 70.4 | 0.8 | 69.9 | 0.9 | 68.7 | 0.8 | 67.0 | 0.7 | 67.3 | 0.7 | 64.9 | 0.7 | 63.8 | 0.7 | 62.2 | 0.6 | 56.3 | 0.4 |
| Income: | ||||||||||||||||||
| Low | 68.9 | 0.6 | 68.7 | 0.6 | 65.7 | 0.6 | 64.0 | 0.6 | 62.7 | 0.6 | 64.9 | 0.6 | 63.0 | 0.5 | 59.8 | 0.5 | 55.7 | 0.5 |
| Medium | 70.0 | 0.6 | 69.1 | 0.6 | 67.7 | 0.6 | 64.9 | 0.6 | 66.2 | 0.6 | 65.2 | 0.6 | 63.1 | 0.6 | 59.4 | 0.5 | 55.9 | 0.5 |
| High | 70.5 | 0.6 | 70.3 | 0.6 | 67.7 | 0.6 | 67.0 | 0.6 | 65.9 | 0.6 | 65.0 | 0.6 | 64.1 | 0.5 | 61.5 | 0.5 | 59.2 | 0.5 |
| Region: | ||||||||||||||||||
| Rural | 69.6 | 0.5 | 70.4 | 0.6 | 69.7 | 0.6 | 67.8 | 0.6 | 66.8 | 0.6 | 66.6 | 0.7 | 64.0 | 0.6 | 61.3 | 0.5 | 55.4 | 0.3 |
| Urban | 69.9 | 0.4 | 68.8 | 0.4 | 65.8 | 0.4 | 64.1 | 0.4 | 64.1 | 0.4 | 64.3 | 0.4 | 63.2 | 0.4 | 59.7 | 0.3 | 59.5 | 0.4 |
| BMI category: | ||||||||||||||||||
| Underweight | 64.9 | 1.1 | 67.3 | 1.2 | 63.8 | 1.2 | 63.5 | 1.4 | 63.9 | 1.5 | 62.5 | 1.4 | 59.2 | 1.1 | 59.0 | 1.2 | 56.3 | 1.0 |
| Normal | 69.9 | 0.4 | 68.9 | 0.4 | 66.9 | 0.4 | 65.5 | 0.4 | 64.6 | 0.5 | 64.7 | 0.4 | 63.6 | 0.4 | 60.6 | 0.4 | 57.0 | 0.4 |
| Overweight/ obesity | 71.5 | 0.7 | 71.3 | 0.7 | 68.3 | 0.7 | 65.2 | 0.6 | 65.8 | 0.6 | 66.1 | 0.6 | 63.9 | 0.5 | 60.0 | 0.5 | 57.2 | 0.4 |
| Total | 69.8 | 0.3 | 69.3 | 0.4 | 67.1 | 0.3 | 65.3 | 0.3 | 64.9 | 0.4 | 65.0 | 0.3 | 63.4 | 0.3 | 60.3 | 0.3 | 57.0 | 0.3 |
The table below shows changing views of the role of amino acids in human nutrition.[4]
| 1954 Indispensable | 1994 Indispensable | 1994 Conditionally indispensable | 1954 Dispensable | 1994 Dispensable |
|---|---|---|---|---|
| Valine | Valine | Glycine | Glycine | Glutamic acid |
| Isoleucine | Isoleucine | Cystine | Cystine | Alanine |
| Leucine | Leucine | Glutamine | Glutamic acid | Serine |
| Lysine | Lysine | Tyrosine | Tyrosine | Aspartic acid |
| Methionine | Methionine | Proline | Proline | Asparagine |
| Phenylalanine | Phenylalanine | Arginine | Arginine | |
| Threonine | Threonine | Taurine | Alanine | |
| Tryptophan | Tryptophan | Serine | ||
| Histidine | Aspartic acid | |||
| Histidine | ||||
| Hydroxyproline | ||||
| Citrulline |
The table below shows estimates made at different times of the protein and energy requirements of children at 1 year of age.[1]
| Year | Protein | Energy | Protein energy | Source |
|---|---|---|---|---|
| g/kg body wt | kcal/kg body wt | % of total energy | ||
| 1948 | 3.3 | 100 | 13.2 | USA |
| 1957 | 2.0 | 100 | 8.0 | FAO |
| 1964 | 2.5 | 100 | 10.0 | USA |
| 1965 | 1.1 | 100 | 4.4 | FAO/WHO |
| 1968 | 1.8 | 100 | 7.2 | USA |
| 1969 | 1.3 | 110 | 4.7 | UK |
| 1973 | 1.27 | 105 | 4.8 | FAO/WHO |
| 1974 | 1.35 | 100 | 5.4 | USA |
Google Schoolar
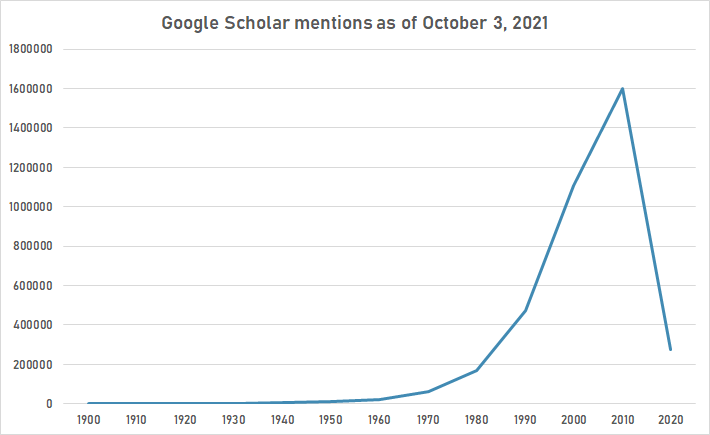
The following table summarizes per-year mentions on Google Scholar as of October 3, 2021.
| Year | "protein" |
|---|---|
| 1900 | 405 |
| 1910 | 925 |
| 1920 | 1,910 |
| 1930 | 3,360 |
| 1940 | 5,070 |
| 1950 | 9,590 |
| 1960 | 21,100 |
| 1970 | 64,500 |
| 1980 | 170,000 |
| 1990 | 472,000 |
| 2000 | 1,110,000 |
| 2010 | 1,600,000 |
| 2020 | 274,000 |
The table below shows dietary requirements of protein (g per kg body weight per day).[5]
| Group | Age (Years) | IOM 2005 | FAO/ WHO/ UNU 1985 | FAO/ WHO/ UNU 2007 |
|---|---|---|---|---|
| Infants | 0.3-0.5 | 1.52 | 1.75 | 1.31 |
| 0.75-1.0 | 1.50 | 1.57 | 1.14 | |
| Children | 1-3 | 1.10 | 1.18 | 1.02 |
| 4-8 | 0.95 | 1.05 | 0.92 | |
| Adolescents | 9-13 | 0.95 | 0.99 | 0.90 |
| 14-18 (boys) | 0.85 | 0.97 | 0.87 | |
| 14-18 (girl) | 0.85 | 0.94 | 0.85 | |
| Adults | >19 | 0.80 | 0.75 | 0.83 |
The table below shows dietary requirements of essential amino acids by healthy human adults.[5]
| EAA | Estimates from N balance experiments Men | Estimates from N balance experiments Women | MIT values (tracer studies) (2000) | IOM (2005) | FAO/ WHO UNU (2007) |
|---|---|---|---|---|---|
| His | - | - | - | 14 | 10 |
| Ile | 10 | 9.17 | 23 | 19 | 20 |
| Leu | 15.7 | 12.1 | 40 | 42 | 39 |
| Lys | 11.4 | 9.07 | 30 | 38 | 30 |
| Met | 2.36 | 3.23 | - | - | - |
| Met+Cys | 15.7 | 11.7 | 13 | 19 | 15 |
| Phe | 4.29 | 4.30 | - | - | - |
| Phe+Tyr | 15.7 | - | 39 | 33 | 25 |
| Thr | 7.14 | 6.25 | 15 | 20 | 15 |
| Trp | 3.57 | 2.80 | 6 | 5 | 4 |
| Val | 11.4 | 10.4 | 20 | 24 | 26 |
| Total | 90.6 | 65.8 | 186 | 214 | 184 |
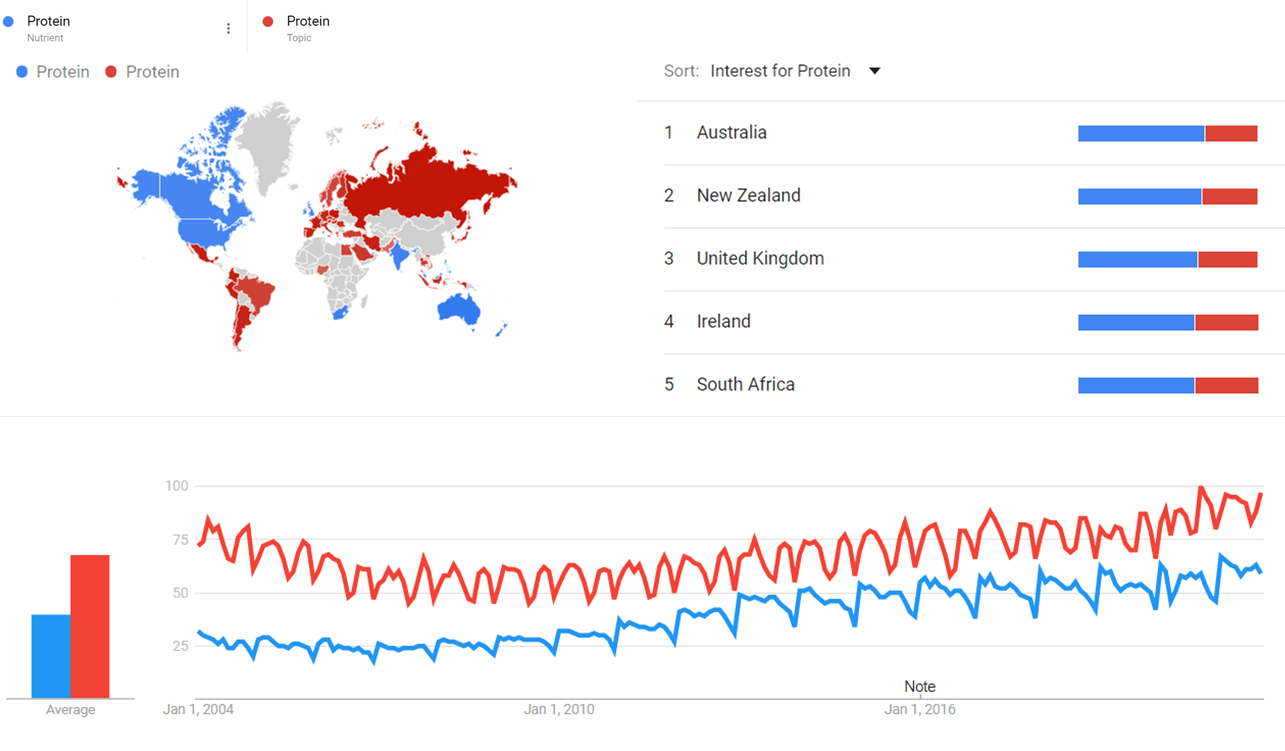
Google Trends
The comparative chart below shows Google Trends data for Protein (Nutrient) and Protein (Topic) from January 2004 to October 2021, when the screenshot was taken. Interest is also ranked by country and displayed on world map.[6]
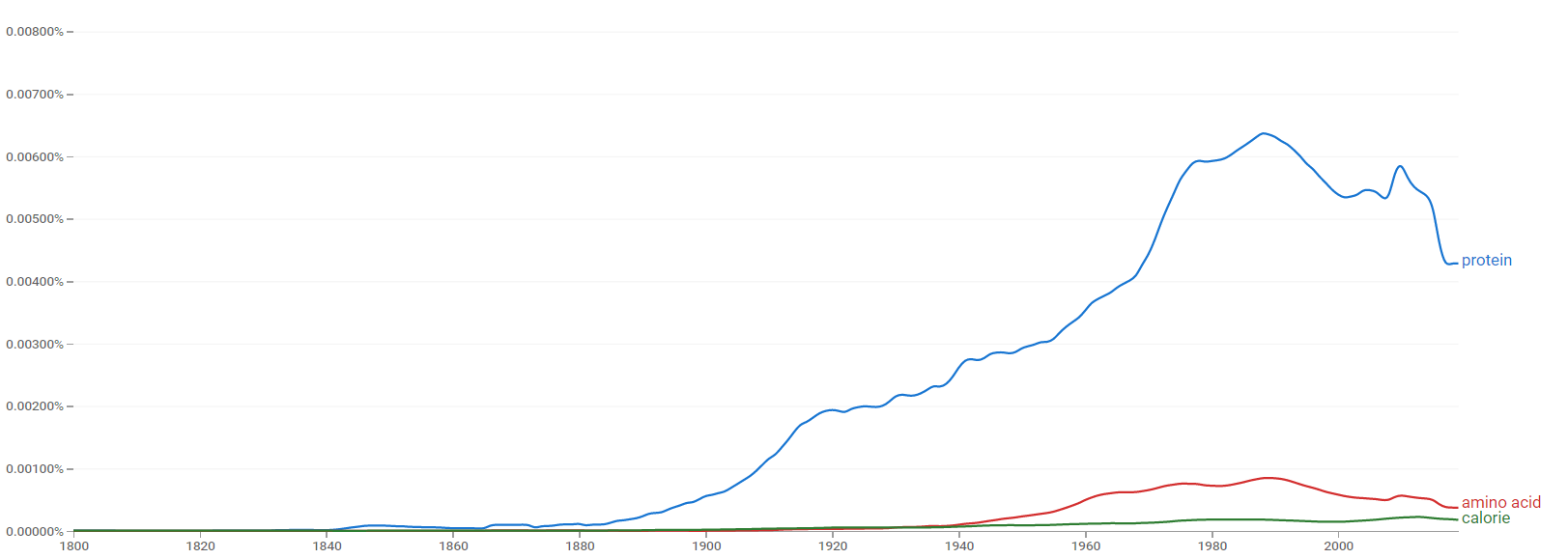
Google Ngram Viewer
The comparative chart below shows Google Ngram Viewer data for Protein, amino acid and calorie from 1800 to 2019.[7]
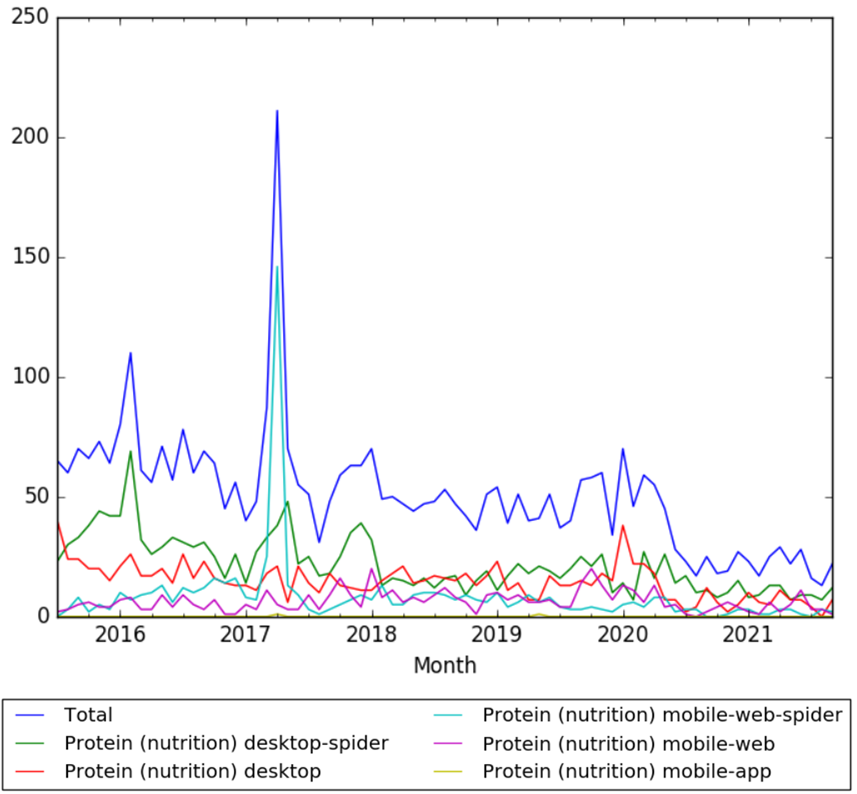
Wikipedia Views
The chart below shows pageviews of the English Wikipedia article Protein, from July 2015 to September 2021.[8]
Full timeline
| Year | Event type | Details |
|---|---|---|
| 1806 | Scientific development | French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet first isolate asparagine in a crystalline form from asparagus juice.[9][10] A non-essential amino acid in humans[11], it is closely related to aspartic acid.[12] Research shows that L-asparagine is able to protect cancer cells from dying due to a loss of glutamine.[13] Rich food sources include dairy, whey, beef, poultry, eggs, fish, seafood, asparagus, potatoes, legumes, nuts, seeds, soy, and whole grains. Poor food sources include most fruits and vegetables.[14] |
| 1810 | Scientific development | English chemist William Hyde Wollaston indirectly discovers cystine, when managing to isolate a crystalline substance from urinary calculi (kidney stones) and calls it “cystic oxide”.[15] Cystine is the oxidized dimer form of the amino acid cysteine It is a non-essential amino acid important for making protein, and for other metabolic functions.[16] |
| 1816 | Scientific development | French physiologist François Magendie demonstrates that dogs would die if fed nothing but carbohydrates or fat, i.e., the principal nonnitrogenous food components.[1] |
| 1819 | Scientific development | The discovery of leucine is attributed to French chemist Joseph Proust who reports its separation from fermented milk curds.[17] Leucine is one of the three branched-chain amino acids (BCAA), along with isoleucine and valine.[18] Like valine, leucine is critical for protein synthesis and muscle repair.[19] Rich food sources include chicken, beef, pork, fish (tuna), tofu, canned beans, milk, cheese, squash seeds, and eggs.[20] |
| 1820 | Scientific development | Glycine is discovered by French chemist Henri Braconnot when he hydrolyzes gelatin by boiling it with sulfuric acid.[21] Glycine is the main amino acid in collagen, the most abundant protein in the body.[22] It is structural of connective tissue, such as bone, skin, ligaments, tendons and cartilage.[23][24] Glycine is found in high-protein foods including meat, fish, eggs, dairy and legumes.[25] |
| 1827 | Scientific development | Aspartic acid (or aspartate) is first discovered by Auguste-Arthur Plisson and Étienne Ossian Henry.[26] It is a non-essential amino acid, meaning that it is readily and naturally synthesized by mammals.[27] D-aspartic acid (D-AA) regulates testosterone synthesis and may act on a stimulatory receptor (NMDA).[28] |
| 1827 | Scientific development | Taurine is first isolated from ox bile by German scientists Friedrich Tiedemann and Leopold Gmelin.[29] It is a type of chemical called an amino sulfonic acid. It occurs naturally in the human body.[30] Taurine is found naturally in meat, fish, dairy products and human milk, and it's also available as a dietary supplement.[31] |
| 1833 | Scientific development | The empirical formula for asparagine is first determined by French chemists Antoine François Boutron Charlard and Théophile-Jules Pelouze. In the same year, German chemist Justus Liebig provides a more accurate formula.[32][33] |
| 1846 | Scientific development | English industrial chemist Edmund Ronalds discovers taurine in human bile.[34] |
| 1846 | Scientific development | Tyrosine is first discovered by German chemist Justus von Liebig in the protein casein from cheese.[35][36] It is one of the 20 standard amino acids that are used by cells to synthesize proteins.[37] Tyrosine is an essential amino acid.[38] It is used to improve alertness, attention and focus.[37] Sources include soy products, chicken, turkey, fish, peanuts, almonds, avocados, bananas, milk, cheese, yogurt, cottage cheese, lima beans, pumpkin seeds, and sesame seeds.[39] |
| 1858 | Scientific development | French chemist Auguste Cahours determines that glycine is an amine of acetic acid.[40] |
| 1859 | Scientific development | German scientists Nikolaus Friedreich and Friedrich August Kekulé von Stradonitz demonstrate that, rather than consisting of cellulose, "amyloid" actually is rich in protein.[41] |
| 1865 | Scientific development | Serine is first obtained by Emil Cramer from silk protein, a particularly rich source. Its name is derived from the Latin for silk, sericum.[42] Serine comes in two forms: L-serine and D-serine.[43] D-serine is used for schizophrenia, cognitive function, and other conditions.[44] It is found in soybeans, nuts (especially peanuts, almonds, and walnuts), eggs, chickpeas, lentils, meat, and fish (especially shellfish).[45] |
| 1866 | Scientific development | Glutamic acid is discovered and identified by German chemist Karl Heinrich Ritthausen.[46] It is a non-essential amino acid that occurs in plants and animals and is formed via protein metabolism.[47] |
| 1869 | Scientific development | German scientists Johannes Wislicenus and Adolf Eugen Fick conduct an experiment in which they measure their urinary nitrogen during and after climbing a Swiss mountain, seeming to demonstrate conclusively that there is no apparent extra breakdown of protein that would be required to provide the energy used in the climb.[1] |
| 1879 | Scientific development | German chemist Ernst Schulze and Johann Barbieri first identify phenylalanine as a constituent of plant proteins.[48][49][50] Phenylalanine is one of the nine essential amino acids. It occurs naturally in many protein-rich foods, such as milk, eggs and meat.[51] |
| 1882 | Scientific development | German chemists Emil Erlenmeyer and Andreas Lipp first synthesize phenylalanine from phenylacetaldehyde, hydrogen cyanide, and ammonia.[52] |
| 1883 | Scientific development | German chemists Ernst Schulze and E. Bosshard isolate L-glutamine from the juice of sugarbeets. L-glutamine is the most common amino acid in human blood and a key component of proteins.[53] The most abundant free amino acid in the body, glutamine is produced in the muscles and is distributed by the blood to the organs that need it.[54] |
| 1886 | Scientific development | Ernst Schulze becomes the first to isolate L-arginine from lupin seedlings. L-arginine is an important amino acid in protein biosynthesis.[53] |
| 1889 | Scientific development | Lysine is first isolated by German biological chemist Ferdinand Heinrich Edmund Drechsel from the protein casein in milk.[55] An essential amino acid, lysine is important for normal growth and muscle turnover and used to form carnitine, a substance found in most cells of the body.[56] |
| 1896 | Scientific development | Histidine is first isolated by German physician Albrecht Kossel and Sven Gustaf Hedin.[57] A semi-essential amino acid (children should obtain it from food)[58], histidine is needed in humans for growth and tissue repair.[59] It is important for maintenance of myelin sheaths that protect nerve cells and is metabolized to the neurotransmitter histamine.[59] Histidine is abundant in meat, fish, poultry, nuts, seeds, and whole grains.[60] |
| 1898 | Scientific development | The first use of the term "amino acid" in the English language dates from this year.[61] |
| 1899 | Scientific development | Cystine is isolated from the horn of a cow.[62] |
| 1890s | Scientific development | The United States Department of Agriculture recommends over 110 g dietary protein per day for working men.[1] |
| 1900 | Recommendation | The official United States Department of Agriculture recommendations around this time are concerned only with providing sufficient protein and energy and how these could be purchased economically.[1] |
| 1900 | Scientific development | German chemist Richard Willstätter first isolates proline[63] and publishes the synthesis of proline from phthalimide propylmalonic ester.[64] A nonessential amino acid[65], proline is helpful for repairing damage to the skin.[66] |
| 1901 | Scientific development | German chemist Hermann Emil Fischer first isolates Valine from casein.[67] A branched-chain essential amino acid with stimulant activity, valine promotes muscle growth and tissue repair. It is a precursor in the penicillin biosynthetic pathway.[68] Valine is found mainly in protein food sources such as meats, fish, soy, and dairy.[69] |
| 1901 | Scientific development | British biochemists Frederick Gowland Hopkins and Sydney William Cole discover tryptophan in the milk derivative casein.[70] An essencial amino acid, tryptophan is vital for a wide variety of metabolic functions that affect the mood, cognition, and behavior.[71] |
| 1902 | Scientific development | The structure of serine is established.[72] |
| 1902 | Scientific development | German chemists Emil Fischer and Fritz Weigert determine lysine's chemical structure by synthesizing it.[73] |
| 1902 | Scientific development | Emil Fischer isolates hydroxyproline from hydrolyzed gelatin.[74] |
| 1902 | Recommendation | W. O. Atwater recommends a dietary intake of 125 g protein per day for the average American adult, because he thinks that U.S. workmen generally work harder than Germans.W. O. Atwater, Principles of Nutrition and Nutritive Value of Food, USDA Farmers’ Bull, no. 142, 1902 |
| 1903 | Scientific development | German chemist Felix Ehrlich discovers isoleucine in hemoglobin.[75] Isoleucine is an amino acid present in most common proteins.[76] It is an essential amino acid[77], critical in physiological functions of the whole body, such as growth, immunity, protein metabolism, fatty acid metabolism and glucose transportation. It can improve the immune system, including immune organs, cells and reactive substances.[78] Isoleucine is abundant in meat, fish, poultry, eggs, cheese, lentils, nuts, and seeds.[79] |
| 1905 | Scientific development | Synthetic isoleucine is originally reported by French chemist Louis Bouveault.[80][81] |
| 1909 | Literature | American biochemist Thomas Burr Osborne publishes The Vegetable Proteins.[82] |
| 1912 | Scientific development | Felix Ehrlich demonstrates that yeast metabolizes the natural amino acids essentially by splitting off carbon dioxide and replacing the amino group with a hydroxyl group. By this reaction, tryptophan gives rise to tryptophol.[83] |
| 1914 | Scientific development | Citrulline is first isolated from watermelon by Japanese researchers Yotaro Koga and Ryo Odake.[84] It is a naturally occurring amino acid.[85] Two commonly seen forms of citrulline seen are citrulline malate and l-citrulline.[86] L-citrulline boosts nitric oxide production in the body. Nitric oxide helps the arteries relax and work better.[87] Citrullus vulgaris (watermelon) is the main source.[88] |
| 1915 | Scientific development | Around this time, with advances in knowledge of vitamins, it begins to be understood that some of the virtues of foods come from their contribution of these accessory factors rather than from protein.[1] |
| 1921 | Scientific development | American scientist John Howard Mueller first isolates methionine,[89] an essential amino acid found in many proteins, including those in foods and those found in the tissues and organs of the body.[90][91] An antioxidant, methionine may help protect the body from damage caused by ionizing radiation.[92] Methionine is abundant in turkey, beef, fish, pork, tofu, milk, cheese, nuts, beans, and whole grains like quinoa.[93] |
| 1923 | Notable comment | Gregor Mendel refers to "the glorification of the albuminous substances" in earlier times, but at the time of writing "the pendulum of enthusiasm about the proteins has swung from one extreme to the other."[1] |
| 1924 | Scientific development | W. C. Rose and coworkers publish a series of landmark papers on amino acid nutrition and metabolism in rats and humans that further define amino acids as nutritionally essential or nonessential, based on nitrogen balance or growth.[94] |
| 1928 | Scientific development | Burger and Coen determine the structure of methionine.[95] |
| 1930 | Recommendation | Fishberg recommends protein restriction for uremic patients.[96] |
| 1935 | Scientific development | Jamaican pediatrician Cicely Williams introduces the term Kwashiorkor two years after publishing the disease's first formal description.[97][98] Kwashiorkor is caused by a lack of protein in the diet.[99] |
| 1936 | Scientific development | Threonine becomes the last of the 20 common proteinogenic amino acids to be discovered. It is discovered by William Cumming Rose,[100] collaborating with Curtis Meyer. The amino acid is named threonine because it is found to be similar in structure to threonic acid, a four-carbon monosaccharide with molecular formula C4H8O5[101] |
| 1939 | Scientific development | Walter Kempner at Duke University introduces the rice diet, low-protein diet to treat kidney disease. It consists in a daily ration of 2,000 calories of moderate amounts of boiled rice, sucrose and dextrose, and a restricted range of fruit, supplemented with vitamins. Sodium and chloride are restricted to 150mg and 200mg respectively. The rice diet shows remarkable effects on control of edema and hypertension.[102][103] |
| 1941 | Recommendation | The Recommended Dietary Allowance (RDA) for protein of 0.8g per kilogram of body weight for adults is established.[104] |
| 1948 | Literature | Melville Sahyun publishes Proteins and Amino Acids in Nutrition, an early book on the topic.[105] |
| 1948 | Scientific development | Borst reports that a protein-free, normal calorie, low salt diet improves uremia and edema in patients with advanced renal failure.[96] |
| 1949 | Scientific development | The first protein to be sequenced is insulin, by Frederick Sanger, who correctly determines its amino acid sequence, thus conclusively demonstrating that proteins consist of linear polymers of amino acids rather than branched chains, colloids, or cyclols.[106] Insulin helps regulate blood sugar levels.[107] |
| 1950 | Scientific development | Protein becomes a matter of nutritional concern as a result of study of the disease, kwashiorkor in young children (usually 6 mo to 2 yr old) first in Africa and then in Jamaica, Central America and else where. This disease, with a high mortality rate, occurs after weaning onto a bulky, low protein diet, and its onset usually follows a bout with diarrhea.[1] |
| 1950 | Scientific development | American biochemist William Cumming Rose identifies methionine and valine as nutritionally essential amino acids for young adults.[108][109] |
| 1950–1975 | Focus trend | Around this time, the work of the Nutrition Division of Food and Agriculture Organization is based on the assumption that "deficiency of protein in the diet is the most serious and widespread problem in the world".[1] |
| 1950–1975 | Focus trend | A downward trend in the successive estimates of protein requirements, particularly for children, occurs over this period.[1] |
| 1954 | Literature | Adelle Davis publishes Let's Eat Right to Keep Fit, which describes the importance of combining "incomplete" proteins to make "complete" proteins, and advises that any incomplete proteins not complemented within one hour could not be used by the body.[110] |
| 1959 | Literature | Anthony Albanese publishes Protein and Amino acid nutrition, which describes the state of knowledge concerning the nutrition of proteins and amino acids.[111] |
| 1959 | Scientific development | Mark Hegsted of the Harvard School of Public Health warns that estimated human protein requirements are excessively reliant on non-human animal studies.[112] |
| 1960 | Recommendation | William C. Rose and Robert L. Wixon from University of Illinois at Urbana-Champaign establish minimum daily requirements of essential amino acids, a discovery made in individuals without kidney disease. This discovery would contribute to the understanding of amino acid metabolism.[96] |
| 1963 | Scientific development | Italian physician Carmelo Giordano applies the concept of high biological value (HBV) protein to the renal diet, in a time when only protein of animal origin is considered HBV. Giordano stresses the need for a specific quality of protein as well as quantity.[96] |
| 1967 | Notable report | The United Nations Advisory Committee on the Application of Science and Technology to Development presents a report to the UN Economic and Social Council entitled 'International action to avert the impending protein crisis'. The preface states that "world food production is falling behind population growth despite all current national, bilateral and international efforts to reverse this trend". Then adds that "adequate protein is also required for the normal maintenance of body tissue and functions, and additionally for growth, maturation, pregnancy, lactation and recovery from injury and disease". The statement continues: "Today there are over 300 million children, who, for lack of sufficient protein and calories suffer grossly retarded physical growth and development, and for many of these, mental development, learning and behaviour may be impaired as well. Protein-calorie deficiencies also directly affect the health and economic productivity of adult populations".[113] |
| 1968 | Notable prediction | It is predicted that, despite the promise of new technologies in the development of unconventional sources of protein food and feed, crops and livestock would continue to be the major sources of protein for human comsumption.[113] |
| 1968 | Program launch | The Food and Agriculture Organization and the International Atomic Energy Agency establish an International Coordinated Research Program on the Use of Nuclear Techniques for Seed Protein Improvement, following recommendations of a panel of experts.[113] |
| 1969 | Scientific development | Goldman et al. report that high protein intakes (from 6g/kg/day) in low birth weight infants are associated with a condition known as late metabolic acidosis.[114][115] |
| 1971 | Literature | Frances Moore Lappé publishes Diet for a Small Planet, which explains how essential amino acids might be obtained from complementary sources in vegetarian nutrition. This book becomes a bestseller.[116] |
| 1971 | Recommendation | The United Nations publishes a document, analyzing the reasons for slow progress in protein food matters and suggesting strategies to avert the protein problem confronting developing countries. Some of these include efforts to balance negative effects of the spread of higher yielding cereal varieties by expanding the 'green revolution' to pulses and oil seed crops, and strengthening the breeding programs for better protein quantity and quality in all important cereals.[113] |
| 1972 | Scientific development | The Protein Advisory Group of the United Nations convenes a symposium at the Food and Agriculture Organization
headquarters in Rome bringing together experts of different fields to review nutritional and food use deficiencies, plant physiology, pathology, and production technology problems which need to be resolved in order to increase the supply of this valuable food item. Six food legumes (dry bean, pigeon pea, cow pea, chickpea, broad bean, pea) and two leguminous oil seeds (peanut, soybean) are identified as priority targets for international and national research efforts.[113] |
| 1973 | Scientific development | Myosin (class I) is discovered in Acanthamoeba by Pollard and Korn.[117] Myosin is a diverse superfamily of motor proteins responsible for actin-based motility and contractility in eukaryotic cells.[118] |
| 1975 | Literature | Both Vogue and American Journal of Nursing carry articles describing the principles and practice of protein combining.[119][120] |
| 1975 | Program launch | The Food and Agriculture Organization and the International Atomic Energy Agency jointly organize a seminar in Sri Lanka to specify the needs for grain legume improvement in South East Asia and to consider the contribution that could be expected from widening genetic variability through mutation induction. It is recognized that many of the currently cultivated legume species still possess a number of 'wild type' characteristics that were beneficial during past natural evolution, but hinder the upgrading of grain production under more intensive farming conditions. South East Asia is an area where many vegetarians rely upon pulses as their main protein source.[113] |
| 1978 | Literature | The American Chemical Society Division of Agricultural and Foods Chemistry publishes Nutritional Improvement of Food and Feed Proteins.[121] |
| 1979 | Scientific development | Isner publishes a report of 17 deaths associated with low-quality liquid protein VLCD, due to heart-related causes.[122] |
| 1982 | Scientific development | Rennie et al. (and later Bennet et al. in 1990[123]) demonstrate that a mixed macronutrient meal is capable of increasing rates of muscle protein synthesis above rates of muscle protein breakdown and that the amino acids contained within the meal are primarily responsible for this increase.[124] |
| 1989 | Literature | C.A. Barth and E. Schlimme publish Milk Proteins: Nutritional, Clinical, Functional and Technological Aspects, which reviews the state of knowledge and progress of research on food proteins, and in particular, milk proteins.[125] |
| 1990 | Literature | Raul A. Wapnir publishes Protein Nutrition and Mineral Absorption, which presents information regarding the mechanisms of protein absorption under normal and pathologic conditions, in addition to reviewing changes that occur at various stages of life.[126] |
| 1993 | Policy | The Protein Digestibility Corrected Amino Acid Score (PDCAAS) is adopted by the US Food and Drug Administration (FDA) and the Food and Agricultural Organization of the United Nations/World Health Organization (FAO/WHO) as "the preferred 'best'" method to determine protein quality. These organizations suggest that other methods for evaluating the quality of protein are inferior.[127] |
| 1994 | Scientific development | Vernon Young and Peter Pellett publish their paper that becomes the definitive contemporary guide to protein metabolism in humans. It also confirms that complementing proteins at meals is totally unnecessary. Thus, people who avoid consuming animal protein do not need to be at all concerned about amino acid imbalances from the plant proteins that make up their usual diets.[128] |
| 1994 | Literature | Zdzisław Sikorski, Bonnie Sun Pan, and Fereidoon Shahidi publish Seafood Proteins, a presentation of the state of knowledge on seafood nitrogenous compounds.[129] |
| 1994 | Scientific development | The Arp2/3 complex (Actin Related Protein 2/3 complex) is named after being identified by affinity chromatography from Acanthamoeba castellanii.[130] The Arp2/3 complex is an essential component of the actin cytoskeleton in eukaryotic cells.[131] |
| 1995 | Literature | Clarence B. Ammerman, David P. Baker, and Austin J. Lewis publish Bioavailability of Nutrients for Animals: Amino Acids, Minerals, Vitamins, which attempts to provide information necessary to formulate diets with appropriate amounts of amino acids, minerals, and vitamins.[132] |
| 1995 | Scientific development | Biolo et al. report that insulin therapy improves protein metabolism.[133] |
| 1996 | Scientific development | Mahe et al. report that soy protein contains a greater proportion of nonessential amino acids than whey protein.[134] |
| 2000 | Scientific development | The term proteopathy is first proposed by Lary Walker and Harry LeVine.[135] It refers to a disease characterized by the production of aberrant conformers of certain proteins that are misfolded and aggregate in a crystallization-like seeding mechanism—changes that lead to a disturbance of their cellular functions and disease.[136] |
| 2000 | Scientific development | Brunton et al. show that the gut has a major effect on amino-acid metabolism.[114] |
| 2000 | Scientific development | Bos et al. conclude that milk proteins are of excellent nutritional value as they have a high metabolic utilization by the organism.[137] |
| 2002 | Recommendation | The U.S. Institute of Medicine sets a Recommended Dietary Allowance (RDA) of 5 mg/kg body weight/day of Tryptophan for adults 19 years and over.[138] |
| 2002 | Notable comment | American physician Dr. John McDougall writes a correction to the American Heart Association for a 2001 publication that questions the completeness of plant proteins, and further asserts that "it is impossible to design an amino acid–deficient diet based on the amounts of unprocessed starches and vegetables sufficient to meet the calorie needs of humans."[139] |
| 2004 | Literature | Rickey Y. Yada publshes Proteins in Food Processing, which reviews how proteins may be used to enhance the nutritional, textural and other qualities of food products.[140] |
| 2005 | Notable comment | Dr. Joel Fuhrman writes:
...plant foods have plenty of protein and you do not have to be a nutritional scientist or dietitian to figure out what to eat and you don’t need to mix and match foods to achieve protein completeness. Any combination of natural foods will supply you with adequate protein, including all eight essential amino acids as well as unessential amino acids.[141] |
| 2005 | Scientific development | Bos et al. show that wheat proteins are the more gluconeogenic because of their high deamination rate and their high glutamine content.[142] Gluconeogenesis is the metabolic process by which glucose is formed from noncarbohydrate sources, such as lactate, amino acids, and glycerol.[143] |
| 2006 | Notable comment | Dr. T. Colin Campbell writes:
We now know that through enormously complex metabolic systems, the human body can derive all the essential amino acids from the natural variety of plant proteins that we encounter every day. It doesn’t require eating higher quantities of plant protein or meticulously planning every meal.[144] |
| 2007 | Recommendation | Isotope studies and reappraisal of nitrogen balance literature using nonlinear regression suggest that the WHO 2007 (Report of a Joint WHO/FAO/UNU Expert Consultation, 2007) estimates of adult protein requirements are too low, by a factor of around 30%.[114] |
| 2007 | Recommendation | The World Health Organization 2007 Technical Report on protein and amino acid requirements in human nutrition states that the best estimate for a population average requirement is 105 mg nitrogen/kg body weight per day, or 0.66 g protein/kg body weight per day.[145] |
| 2007 | Scientific development | Bauchart et al. report having identified in the stomach effluent a large number of peptides deriving from actin and myosin (the main muscle proteins) after meat or fish consumption.[146] |
| 2008 | Scientific development | Pikachurin is first discovered in Japan by Shigeru Sato et al. and named after Pikachu, a species of the Pokémon franchise.[147] An extracellular matrix-like retinal protein, its name is inspired by Pikachu's "lightning-fast moves and shocking electric effects".[148] |
| 2009 | Notable comment | The American Dietetic Association writes:
Plant protein can meet protein requirements when a variety of plant foods is consumed and energy needs are met. Research indicates that an assortment of plant foods eaten over the course of a day can provide all essential amino acids and ensure adequate nitrogen retention and use in healthy adults, thus, complementary proteins do not need to be consumed at the same meal.[149] |
| 2010 | Scientific development | A study from the University of Copenhagen concludes that protein requirements should be based on criteria related to long-term health and well-being, rather than on nitrogen balance alone.[150][145] |
| 2011 | Scientific development | A review concludes that a "long-term effect of high-protein diets is neither consistent nor conclusive."[151] |
| 2011 | Literature | G. O. Phillips and P. A. Williams publish their Handbook of Food Proteins, which provides an overview of the characteristics, functionalities and applications of different proteins of importance to the food industry.[152] |
| 2011 | Scientific development | Rubinsztein et al. report that autophagy eliminates protein aggregates that are toxic to the cell, thus mediating cytoprotection.[153] |
| 2012 | Scientific development | Study shows that exercise regulates protein synthesis.[154] |
| 2013 | Scientific development | Bollwein et al. report that protein intake may be more evenly distributed throughout the day in the frail elderly population than in healthy adults.[155] |
| 2014 | Scientific development | A review notes that high-protein diets from animal sources should be handled with caution.[156] |
| 2014 | Scientific development | Researchers report that resistance in older people to postprandial anabolic stimulation by dietary protein can be overcome by supplying daily protein in the form of protein-rich meals.[157][158] |
| 2015 | Recommendation | Layman et al. recommend daily allowance for dietary protein intake by an adult person at 0.8 g/kg per day, irrespective of overall energy intake.[159] |
| 2015–2020 | Recommendation | The 2015–2020 Dietary Guidelines for Americans (DGA) recommends that men and teenage boys increase their consumption of fruits, vegetables and other under-consumed foods, and that a means of accomplishing this would be to reduce overall intake of protein foods.[160] |
| 2016 | Notable comment | The American Heart Association states:
You don’t need to eat foods from animals to have enough protein in your diet. Plant proteins alone can provide enough of the essential and non-essential amino acids, as long as sources of dietary protein are varied and caloric intake is high enough to meet energy needs. Whole grains, legumes, vegetables, seeds and nuts all contain both essential and non-essential amino acids. You don’t need to consciously combine these foods (“complementary proteins”) within a given meal.[161] |
| 2017 | Scientific development | A review indicates that a high-protein diet may contribute to life-long risk of kidney damage, including chronic kidney disease.[162] |
| 2020 | Scientific development | A review finds that a high-protein diet does not significantly improve blood pressure and glycemic control in people with diabetes.[163] |
Meta information on the timeline
How the timeline was built
The initial version of the timeline was written by User:Sebastian.
Funding information for this timeline is available.
Feedback and comments
Feedback for the timeline can be provided at the following places:
- FIXME
What the timeline is still missing
Timeline update strategy
See also
External links
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Carpenter, KJ (July 1986). "The history of enthusiasm for protein.". The Journal of nutrition. 116 (7): 1364–70. PMID 3528432. doi:10.1093/jn/116.7.1364.
- ↑ 2.0 2.1 Semba, Richard D. (2016). "The Rise and Fall of Protein Malnutrition in Global Health". Annals of Nutrition & Metabolism. 69 (2): 79–88. ISSN 1421-9697. doi:10.1159/000449175.
- ↑ Zhao, Jian; Su, Chang; Wang, Huijun; Wang, Zhihong; Wang, Yun; Zhang, Bing (24 January 2018). "Secular Trends in Energy and Macronutrient Intakes and Distribution among Adult Females (1991–2015): Results from the China Health and Nutrition Survey". Nutrients. 10 (2): 115. doi:10.3390/nu10020115.
- ↑ Dupont, Christophe (1 June 2003). "Protein requirements during the first year of life". The American Journal of Clinical Nutrition. 77 (6): 1544S–1549S. doi:10.1093/ajcn/77.6.1544S.
- ↑ 5.0 5.1 Wu, Guoyao (2016). "Dietary protein intake and human health". Food & Function. pp. 1251–1265. doi:10.1039/C5FO01530H. Retrieved 19 October 2021.
- ↑ "Protein". Google Trends. Retrieved 3 October 2021.
- ↑ "Protein, amino acid and calorie". books.google.com. Retrieved 3 October 2021.
- ↑ "Protein". wikipediaviews.org. Retrieved 3 October 2021.
- ↑ Vauquelin LN, Robiquet PJ (1806). "La découverte d'un nouveau principe végétal dans le suc des asperges". Annales de Chimie (in français). 57: 88–93. hdl:2027/nyp.33433062722578.
- ↑ Plimmer RH (1912) [1908]. Plimmer RH, Hopkins FG, eds. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 112. Retrieved January 18, 2010.
- ↑ "Asparagine". pubchem.ncbi.nlm.nih.gov. Retrieved 7 October 2021.
- ↑ "asparagine | chemical compound". Encyclopedia Britannica. Retrieved 7 October 2021.
- ↑ "Asparagus and Breast Cancer: Does Asparagus Help Fight or Spread It?". Healthline. 19 March 2019. Retrieved 7 October 2021.
- ↑ "Low-Asparagine Diet Could Slow Breast Cancer's Spread". genengnews.com. Retrieved 7 October 2021.
- ↑ "Cysteine: an Essential Inessential Amino Acid" (PDF). eurpepsoc.com.
- ↑ "Cysteine - Health Encyclopedia - University of Rochester Medical Center". www.urmc.rochester.edu. Retrieved 2 October 2021.
- ↑ "Leucine". www.drugfuture.com.
- ↑ Mero, Antti (1999). "Leucine Supplementation and Intensive Training:". Sports Medicine. 27 (6): 347–358. doi:10.2165/00007256-199927060-00001.
- ↑ "10 Healthy High Leucine Foods". Healthline. 9 June 2021. Retrieved 4 October 2021.
- ↑ "Top 10 Foods Highest in Leucine". myfooddata. Retrieved 7 October 2021.
- ↑ Plimmer, R.H.A. (1912) [1908]. Plimmer, R.H.A.; Hopkins, F.G., eds. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 82. Retrieved January 18, 2010.
- ↑ Razak, Meerza Abdul; Begum, Pathan Shajahan; Viswanath, Buddolla; Rajagopal, Senthilkumar (2017). "Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review". Oxidative Medicine and Cellular Longevity. 2017: 1–8. doi:10.1155/2017/1716701.
- ↑ "Top 9 Benefits and Uses of Glycine". Healthline. 12 December 2018. Retrieved 4 October 2021.
- ↑ "Collagen — What Is It and What Is It Good For?". Healthline. 5 May 2020. Retrieved 4 October 2021.
- ↑ Breus, Dr Michael (23 July 2018). "The Connection Between Glycine and Sleep". The Sleep Doctor. Retrieved 7 October 2021.
- ↑ Berzelius (friherre), Jöns Jakob (1839). Traité de chimie (in français). A. Wahlen et Cie.
- ↑ "Amyloid". www.sciencedirect.com. Retrieved 7 October 2021.
- ↑ Frank, Kurtis; Patel, Kamal; Lopez, Gregory; Willis, Bill (17 September 2019). "D-Aspartic Acid Research Analysis". Retrieved 7 October 2021.
- ↑ Tiedemann F, Gmelin L (1827). "Einige neue Bestandtheile der Galle des Ochsen". Annalen der Physik. 85 (2): 326–37. doi:10.1002/andp.18270850214.
- ↑ "TAURINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews". www.webmd.com. Retrieved 9 October 2021.
- ↑ "Does your energy drink contain taurine?". Mayo Clinic. Retrieved 8 October 2021.
- ↑ Boutron-Charlard; Pelouze (1833). "Ueber das Asparamid (Asparagin des Herrn Robiquet) und die Asparamidsäure" [On asparamide (the asparagine of Mr. Robiquet) and aspartic acid]. Annalen der Chemie (in Deutsch). 6: 75–88. doi:10.1002/jlac.18330060111. The empirical formula of asparagine appears on p. 80.
- ↑ Liebig, Justus (1834). "Ueber die Zusammensetzung des Asparamids und der Asparaginsäure". Annalen der Physik und Chemie. 107 (14): 220–224. doi:10.1002/andp.18341071405.
- ↑ Ronalds BF (2019). "Bringing Together Academic and Industrial Chemistry: Edmund Ronalds' Contribution". Substantia. 3 (1): 139–152.
- ↑ "Tyrosine". The Columbia Electronic Encyclopedia, 6th ed. Infoplease.com — Columbia University Press. 2007. Retrieved 1 October 2021.
- ↑ Harper D (2001). "Tyrosine". Online Etymology Dictionary. Retrieved 2008-04-20.
- ↑ 37.0 37.1 "Tyrosine: Benefits, Side Effects and Dosage". Healthline. 1 February 2018. Retrieved 4 October 2021.
- ↑ "Amino Acids - Tyrosine". www.biology.arizona.edu. Retrieved 8 October 2021.
- ↑ "Tyrosine Information | Mount Sinai - New York". Mount Sinai Health System. Retrieved 8 October 2021.
- ↑ Cahours, A. (1858). "Recherches sur les acides amidés" [Investigations into aminated acids]. Comptes Rendus (in français). 46: 1044–1047.
- ↑ Sipe JD, Cohen AS (June 2000). "Review: history of the amyloid fibril". Journal of Structural Biology. 130 (2–3): 88–98. PMID 10940217. doi:10.1006/jsbi.2000.4221.
- ↑ "'Journal für praktische Chemie : practical applications and applied chemistry ; covering all aspects of applied chemistry. 96. 1865' - Digitalisat | MDZ". www.digitale-sammlungen.de. Retrieved 7 October 2021.
- ↑ "SERINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews". www.webmd.com. Retrieved 7 October 2021.
- ↑ "SERINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews". www.webmd.com. Retrieved 8 October 2021.
- ↑ "Serine - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 7 October 2021.
- ↑ R. H. A. Plimmer (1912) [1908]. R. H. A. Plimmer; F. G. Hopkins, eds. The Chemical Constitution of the Protein. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 114. Retrieved June 3, 2012.
- ↑ "Glutamic acid". pubchem.ncbi.nlm.nih.gov.
- ↑ Proteins, Enzymes, Genes: The Interplay of Chemistry and Biology. Yale University Press. ISBN 978-0-300-15359-0.
- ↑ "Phenylalanine hydroxylase and phenylketonuria". 2016. doi:10.13140/RG.2.1.3339.3529.
- ↑ Advances in Protein Chemistry. Academic Press. 1 January 1955. ISBN 978-0-08-058183-5.
- ↑ "Phenylalanine in diet soda: Is it harmful?". Mayo Clinic. Retrieved 4 October 2021.
- ↑ Thorpe, Sir Edward (10 December 2019). A Dictionary of Applied Chemistry (Volume III). Alpha Editions. ISBN 978-93-5395-007-1.
- ↑ 53.0 53.1 "L-Glutamine". American Chemical Society. Retrieved 2 October 2021.
- ↑ "GLUTAMINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews". www.webmd.com. Retrieved 4 October 2021.
- ↑ Drechsel E (1889). "Zur Kenntniss der Spaltungsprodukte des Caseïns" [[Contribution] to [our] knowledge of the cleavage products of casein]. Journal für Praktische Chemie. 2nd series (in Deutsch). 39: 425–429. doi:10.1002/prac.18890390135. On p. 428, Drechsel presented an empirical formula for the chloroplatinate salt of lysine – C8H16N2O2Cl2•PtCl4 + H2O – but he later admitted that this formula was wrong because the salt's crystals contained ethanol instead of water. See: Drechsel E (1891). "Der Abbau der Eiweissstoffe" [The disassembly of proteins]. Archiv für Anatomie und Physiologie (in Deutsch): 248–278.; Drechsel E. "Zur Kenntniss der Spaltungsproducte des Caseïns" [Contribution] to [our] knowledge of the cleavage products of casein] (in Deutsch): 254–260.
From p. 256:] " … die darin enthaltene Base hat die Formel C6H14N2O2. Der anfängliche Irrthum ist dadurch veranlasst worden, dass das Chloroplatinat nicht, wie angenommen ward, Krystallwasser, sondern Krystallalkohol enthält, … " ( … the base [that's] contained therein has the [empirical] formula C6H14N2O2. The initial error was caused by the chloroplatinate containing not water in the crystal (as was assumed), but ethanol … )
- ↑ "4 Impressive Health Benefits of Lysine". Healthline. 6 October 2018. Retrieved 8 October 2021.
- ↑ Vickery, Hubert Bradford; Leavenworth, Charles S. (1928-08-01). "On the Separation of Histidine and Arginine" (PDF). Journal of Biological Chemistry. 78 (3): 627–635. ISSN 0021-9258. doi:10.1016/S0021-9258(18)83967-9
 .
.
- ↑ "Health Topics A-Z". PeaceHealth. Retrieved 10 October 2021.
- ↑ 59.0 59.1 "Histidine". pubchem.ncbi.nlm.nih.gov. Retrieved 8 October 2021.
- ↑ "Essential amino acids: Definition, benefits, and foods". www.medicalnewstoday.com. 21 January 2019. Retrieved 10 October 2021.
- ↑ "amino- | Etymology, origin and meaning of prefix amino- by Etymonline". www.etymonline.com. Retrieved 1 November 2021.
- ↑ "cystine". Encyclopædia Britannica. 2007. Encyclopædia Britannica Online. 27 July 2007
- ↑ R.H.A. Plimmer (1912) [1908], R.H.A. Plimmer & F.G. Hopkins, ed., The chemical composition of the proteins, Monographs on biochemistry, Part I. Analysis (2nd ed.), London: Longmans, Green and Co., p. 130, retrieved September 20, 2010
- ↑ "Proline - Cas 147-85-3 - nonessential amino acid". web.archive.org. 27 November 2015. Retrieved 4 October 2021.
- ↑ "Proline - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 4 October 2021.
- ↑ "The Amino Acid that Heals Wounds & Supports Digestion". Dr. Axe.
- ↑ "Valine". Encyclopædia Britannica Online. Retrieved 1 October 2021.
- ↑ "Valine". go.drugbank.com. Retrieved 4 October 2021.
- ↑ "Valine - Health Encyclopedia - University of Rochester Medical Center". www.urmc.rochester.edu. Retrieved 4 October 2021.
- ↑ "L-Tryptophan". American Chemical Society. Retrieved 4 October 2021.
- ↑ "Top Foods High in Tryptophan". WebMD. Retrieved 4 October 2021.
- ↑ "Serine | Encyclopedia.com". www.encyclopedia.com. Retrieved 7 October 2021.
- ↑ Fischer, Emil; Weigert, Fritz (July 1902). "Synthese der α, ϵ-Diaminocapronsäure (Inactives Lysin)". Berichte der deutschen chemischen Gesellschaft. 35 (3): 3772–3778. doi:10.1002/cber.190203503211.
- ↑ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer; F.G. Hopkins, eds. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 132. Retrieved January 18, 2010.
- ↑ "Isoleucine Explained". everything.explained.today. Retrieved 4 October 2021.
- ↑ "isoleucine | chemical compound". Encyclopedia Britannica. Retrieved 4 October 2021.
- ↑ "Amino Acids - Alanine". www.biology.arizona.edu. Retrieved 4 October 2021.
- ↑ Gu, Changsong; Mao, Xiangbing; Chen, Daiwen; Yu, Bing; Yang, Qing (27 June 2019). "Isoleucine Plays an Important Role for Maintaining Immune Function". Current Protein & Peptide Science. 20 (7): 644–651. doi:10.2174/1389203720666190305163135.
- ↑ "Essential amino acids: Definition, benefits, and foods". www.medicalnewstoday.com. 21 January 2019. Retrieved 10 October 2021.
- ↑ texte, Académie des sciences (France) Auteur du (January 1905). "Comptes rendus hebdomadaires des séances de l'Académie des sciences / publiés... par MM. les secrétaires perpétuels". Gallica.
- ↑ "[:it]Isoleucina: formula, storia, biosintesi, presenza in natura, funzioni[:]". Un Mondo Ecosostenibile. 30 November 2020. Retrieved 1 October 2021.
- ↑ "Thomas Burr Osborne". prabook.com. Retrieved 22 October 2021.
- ↑ Jackson RW (1930). "A synthesis of tryptophol" (PDF). Journal of Biological Chemistry. 88 (3): 659–662. doi:10.1016/S0021-9258(18)76755-0
 .
.
- ↑ Fragkos, Konstantinos C.; Forbes, Alastair (September 2011). "Was citrulline first a laxative substance? The truth about modern citrulline and its isolation" (PDF). Nihon Ishigaku Zasshi. [Journal of Japanese History of Medicine]. 57 (3): 275–292. ISSN 0549-3323. PMID 22397107.
- ↑ "L-CITRULLINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews". www.webmd.com. Retrieved 7 October 2021.
- ↑ "Citrulline Malate: The Most Mis-Used Weapon in Aerobic and Anaerobic Performance". Just Fly Sports Performance. Retrieved 7 October 2021.
- ↑ Miller, Kelli. "L-citrulline: Uses and Risks". WebMD. Retrieved 11 October 2021.
- ↑ "Citrulline Food Sources & Recommended Dietary Intake". Nootriment - Health Supplement Reviews and Research. 30 March 2016. Retrieved 8 October 2021.
- ↑ Pappenheimer AM (1987). "A Biographical Memoir of John Howard Mueller" (PDF). Washington D.C.: National Academy of Sciences.
- ↑ "Methionine: Functions, Food Sources and Side Effects". Healthline. 13 April 2018. Retrieved 4 October 2021.
- ↑ Parker, Stewart F.; Funnell, Nicholas P.; Shankland, Kenneth; Kabova, Elena A.; Häußner, Thomas; Hasselbach, Hans-Joachim; Braune, Sascha; Kobler, Christoph; Albers, Peter W. (11 January 2021). "Structure and spectroscopy of methionyl-methionine for aquaculture". Scientific Reports. 11 (1): 458. ISSN 2045-2322. doi:10.1038/s41598-020-80385-z.
- ↑ "Methionine - Health Encyclopedia - University of Rochester Medical Center". www.urmc.rochester.edu. Retrieved 10 October 2021.
- ↑ "Top 10 Foods Highest in Methionine". myfooddata. Retrieved 11 October 2021.
- ↑ Hou, Yongqing; Wu, Guoyao (1 November 2018). "Nutritionally Essential Amino Acids". Advances in Nutrition. 9 (6): 849–851. doi:10.1093/advances/nmy054.
- ↑ "LYSINE AND METHIONINE CONTENTS OF DRY-MILLED GRAIN SORGHUM FRACTIONS ALTERED BY YEAST GROWTH" (PDF).
- ↑ 96.0 96.1 96.2 96.3 Byham-Gray, Laura D.; Burrowes, Jerrilynn D.; Chertow, Glenn M. (15 May 2008). Nutrition in Kidney Disease. Springer Science & Business Media. ISBN 978-1-59745-032-4.
- ↑ Williams, C D (1 July 1983). "Fifty years ago. Archives of Diseases in Childhood 1933. A nutritional disease of childhood associated with a maize diet.". Archives of Disease in Childhood. 58 (7): 550–560. doi:10.1136/adc.58.7.550.
- ↑ Williams, CicelyD. (November 1935). "KWASHIORKOR". The Lancet. 226 (5855): 1151–1152. doi:10.1016/S0140-6736(00)94666-X.
- ↑ "Kwashiorkor: Causes, Symptoms, and Diagnosis". Healthline. 15 August 2012. Retrieved 4 October 2021.
- ↑ A Dictionary of scientists. Daintith, John., Gjertsen, Derek. Oxford: Oxford University Press. 1999. p. 459. ISBN 9780192800862.
- ↑ Meyer, Curtis (20 July 1936). "The Spatial Configuation of Alpha-Amino-Beta-Hydroxy-n-Butyric Acid" (PDF). Journal of Biological Chemistry. 115 (3): 721–729. doi:10.1016/S0021-9258(18)74711-X.
- ↑ Kempner, Walter (1946). "Some Effects of the Rice Diet Treatment of Kidney Disease and Hypertension". Bulletin of the New York Academy of Medicine. 22 (7): 358–70. PMC 1871537
 . PMID 19312487.
. PMID 19312487.
- ↑ Kempner, Walter (1948). "Treatment of hypertensive vascular disease with rice diet". The American Journal of Medicine. 4 (4): 545–77. PMID 18909456. doi:10.1016/0002-9343(48)90441-0.
- ↑ Rodriguez, Nancy R. (30 April 2021). "Role of Meat in Healthy Eating Patterns: Considerations for Protein Quantity and Protein Quality". Meat and Muscle Biology. 4 (2). ISSN 2575-985X. doi:10.22175/mmb.11687.
- ↑ Sahyun, Melville (1 March 2007). Proteins and Amino Acids in Nutrition. Read Books. ISBN 978-1-4067-4730-0.
- ↑ Sanger, F. (1 January 1949). "The terminal peptides of insulin". Biochemical Journal. 45 (5): 563–574. doi:10.1042/bj0450563.
- ↑ "Diabetes treatment: Using insulin to manage blood sugar". Mayo Clinic. Retrieved 11 October 2021.
- ↑ Rose, W C (November 1968). "II. The sequence of events leading to the establishment of the amino acid needs of man.". American Journal of Public Health and the Nations Health. 58 (11): 2020–2027. doi:10.2105/AJPH.58.11.2020.
- ↑ Wu, Guoyao (2016). "Dietary protein intake and human health". Food & Function. 7 (3): 1251–1265. doi:10.1039/C5FO01530H.
- ↑ Davis, Adelle (1954). Let's Eat Right to Keep Fit. Harcourt, Brace. ISBN 4-87187-961-5.
- ↑ Albanese, Anthony (2 December 2012). Protein and Amino acid nutrition. Elsevier. ISBN 978-0-323-14445-2.
- ↑ Webb, Geoff. "The protein gap – nutritional science's biggest error". The Conversation. Retrieved 3 November 2021.
- ↑ 113.0 113.1 113.2 113.3 113.4 113.5 Gottschalk, W.; Müller, H. P. (3 November 2011). Seed Proteins: Biochemistry, Genetics, Nutritive Value. Springer Netherlands. ISBN 978-94-009-6803-5.
- ↑ 114.0 114.1 114.2 Pencharz, P B (May 2010). "Protein and energy requirements for 'optimal' catch-up growth". European Journal of Clinical Nutrition. 64 (S1): S5–S7. doi:10.1038/ejcn.2010.39.
- ↑ "AMINO ACID REQUIREMENTS OF ADULT MAN". Nutrition Reviews. 14 (8): 232–235. 27 April 2009. doi:10.1111/j.1753-4887.1956.tb01591.x.
- ↑ Belasco, Warren James (1989). Appetite for change : how the counterculture took on the food industry, 1966-1988 (1st ed.). New York: Pantheon Books. ISBN 0394543998.
- ↑ Oosawa, Fumio (16 November 2018). "Discovery of myosin I and Pollard-san". Biophysical Reviews. 10 (6): 1481–1482. ISSN 1867-2450. doi:10.1007/s12551-018-0485-5.
- ↑ Ouyang, Zhaohui; Zhao, Shuangshuang; Yao, Su; Wang, Jing; Cui, Yanqin; Wei, Ke; Jiu, Yaming (2021). "Multifaceted Function of Myosin-18, an Unconventional Class of the Myosin Superfamily". Frontiers in Cell and Developmental Biology. 9: 2. ISSN 2296-634X. doi:10.3389/fcell.2021.632445.
- ↑ Judith S. Stern (1975) "How to stay well on a vegetarian diet and save money too!", Vogue 165(2):150,1
- ↑ Eleanor R. Williams (1975) Making Vegetarian Diets Nutritious, }}w|American Journal of Nursing}} 75(12):2168–73 from JSTOR
- ↑ Ill.), Symposium on Improvement of Protein Nutritive Quality of Foods and Feeds (1977, Chicago; Chemistry, American Chemical Society Division of Agricultural and Foods (October 1978). Nutritional Improvement of Food and Feed Proteins. Springer US. ISBN 978-0-306-40026-1.
- ↑ Isner, J M; Sours, H E; Paris, A L; Ferrans, V J; Roberts, W C (December 1979). "Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval.". Circulation. 60 (6): 1401–1412. ISSN 0009-7322. PMID 498466. doi:10.1161/01.CIR.60.6.1401.
- ↑ Bennet, W. M.; Connacher, A. A.; Scrimgeour, C. M.; Rennie, M. J. (1990). "The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange". European Journal of Clinical Investigation. 20 (1): 41–50. ISSN 1365-2362. doi:10.1111/j.1365-2362.1990.tb01789.x.
- ↑ Rennie, M. J.; Edwards, R. H. T.; Halliday, D.; Matthews, D. E.; Wolman, S. L.; Millward, D. J. (1 December 1982). "Muscle Protein Synthesis Measured by Stable Isotope Techniques in Man: The Effects of Feeding and Fasting". Clinical Science. 63 (6): 519–523. ISSN 0143-5221. doi:10.1042/cs0630519.
- ↑ Barth, C. A.; Schlimme, E. (1989). Milk Proteins: Nutritional, Clinical, Functional and Technological Aspects. Steinkopff. ISBN 978-3-7985-0786-9.
- ↑ Wapnir, Raul A. (25 September 1990). Protein Nutrition and Mineral Absorption. CRC Press. ISBN 978-0-8493-5227-0.
- ↑ Boutrif, E., Food Quality and Consumer Protection Group, Food Policy and Nutrition Division, FAO, Rome: "Recent Developments in Protein Quality Evaluation" Food, Nutrition and Agriculture, Issue 2/3, 1991
- ↑ Young VR, Pellett PL (1994). "Plant proteins in relation to human protein and amino acid nutrition". The American Journal of Clinical Nutrition. 59 (5 Suppl): 1203S–1212S. PMID 8172124. doi:10.1093/ajcn/59.5.1203s.
- ↑ SIKORSKI, Zdzisław; Pan, Bonnie Sun; Shahidi, Fereidoon (1994). Seafood Proteins. Springer US. ISBN 978-0-412-98481-5.
- ↑ Machesky, L M; Atkinson, S J; Ampe, C; Vandekerckhove, J; Pollard, T D (1 October 1994). "Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose.". Journal of Cell Biology. 127 (1): 107–115. doi:10.1083/jcb.127.1.107.
- ↑ Dyche Mullins, R; Pollard, Thomas D (1 April 1999). "Structure and function of the Arp2/3 complex". Current Opinion in Structural Biology. 9 (2): 244–249. ISSN 0959-440X. doi:10.1016/S0959-440X(99)80034-7.
- ↑ Ammerman, Clarence B.; Baker, David P.; Lewis, Austin J. (19 July 1995). Bioavailability of Nutrients for Animals: Amino Acids, Minerals, Vitamins. Elsevier. ISBN 978-0-08-052787-1.
- ↑ Biolo, G.; Fleming, R. Y. Declan; Wolfe, R. R. (1 February 1995). Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. pp. 811–819.
- ↑ Mahé, S; Roos, N; Benamouzig, R; Davin, L; Luengo, C; Gagnon, L; Gaussergès, N; Rautureau, J; Tomé, D (1 April 1996). "Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: the influence of the nature and quantity of the protein". The American Journal of Clinical Nutrition. 63 (4): 546–552. doi:10.1093/ajcn/63.4.546.
- ↑ Walker LC, LeVine H (2000). "The cerebral proteopathies". Neurobiology of Aging. 21 (4): 559–61. PMID 10924770. doi:10.1016/S0197-4580(00)00160-3.
- ↑ "Proteopathy - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 22 October 2021.
- ↑ Bos, Cécile; Gaudichon, Claire; Tomé, Daniel (1 April 2000). "Nutritional and Physiological Criteria in the Assessment of Milk Protein Quality for Humans". Journal of the American College of Nutrition. 19 (sup2): 191S–205S. ISSN 0731-5724. doi:10.1080/07315724.2000.10718068.
- ↑ Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768. ISBN 978-0-309-08525-0. doi:10.17226/10490.
- ↑ McDougall, J (25 Jun 2002). "Plant foods have a complete amino acid composition". Circulation. 105 (25): e197. PMID 12082008. doi:10.1161/01.CIR.0000018905.97677.1F.
- ↑ Yada, Rickey Y. (13 November 2017). Proteins in Food Processing. Woodhead Publishing. ISBN 978-0-08-100729-7.
- ↑ Fuhrman, Joel (2005). Eat to Live
 . Little Brown. p. 137. ISBN 9780316735506.
. Little Brown. p. 137. ISBN 9780316735506.
- ↑ Bos, Cécile; Juillet, Barbara; Fouillet, Hélène; Turlan, Lucie; Daré, Sophie; Luengo, Catherine; N’tounda, Rufin; Benamouzig, Robert; Gausserès, Nicolas; Tomé, Daniel; Gaudichon, Claire (1 January 2005). "Postprandial metabolic utilization of wheat protein in humans". The American Journal of Clinical Nutrition. 81 (1): 87–94. doi:10.1093/ajcn/81.1.87.
- ↑ "Gluconeogenesis - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 10 December 2021.
- ↑ Campbell, T. Colin; Campbell, Thomas M. (2006). The China Study
 . BenBella Books. p. 31. ISBN 1935251007.
. BenBella Books. p. 31. ISBN 1935251007.
- ↑ 145.0 145.1 Schönfeldt, Hettie Carina; Hall, Nicolette Gibson (August 2012). "Dietary protein quality and malnutrition in Africa". British Journal of Nutrition. 108 (S2): S69–S76. ISSN 0007-1145. doi:10.1017/S0007114512002553.
- ↑ Bauchart, Caroline; Morzel, Martine; Chambon, Christophe; Mirand, Philippe Patureau; Reynès, Christelle; Buffière, Caroline; Rémond, Didier (December 2007). "Peptides reproducibly released by in vivo digestion of beef meat and trout flesh in pigs". British Journal of Nutrition. 98 (6): 1187–1195. ISSN 1475-2662. doi:10.1017/S0007114507761810.
- ↑ Sato, Shigeru; Omori, Yoshihiro; Katoh, Kimiko; Kondo, Mineo; Kanagawa, Motoi; Miyata, Kentaro; Funabiki, Kazuo; Koyasu, Toshiyuki; Kajimura, Naoko; Miyoshi, Tomomitsu; Sawai, Hajime; Kobayashi, Kazuhiro; Tani, Akiko; Toda, Tatsushi; Usukura, Jiro; Tano, Yasuo; Fujikado, Takashi; Furukawa, Takahisa (August 2008). "Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation". Nature Neuroscience. 11 (8): 923–931. doi:10.1038/nn.2160.
- ↑ "Lightning-Fast Vision Protein Named After Pikachu". InventorSpot.com. Retrieved 10 December 2021.
- ↑ Craig, WJ; Mangels, AR (July 2009). "Position of the American Dietetic Association: Vegetarian Diets" (PDF). Journal of the American Dietetic Association. 109 (7): 1267–1268. PMID 19562864. doi:10.1016/j.jada.2009.05.027. Retrieved 13 January 2017.
- ↑ Jakobsen, Lene H.; Kondrup, Jens; Zellner, Maria; Tetens, Inge; Roth, Erich (June 2011). "Effect of a high protein meat diet on muscle and cognitive functions: A randomised controlled dietary intervention trial in healthy men". Clinical Nutrition. 30 (3): 303–311. doi:10.1016/j.clnu.2010.12.010.
- ↑ Lepe M, Bacardi Gascon M, Jimenez Cruz. (2011). A: Long-term efficacy of high-protein diets: a systematic review. Nutr Hosp 26: 1256-1259.
- ↑ Phillips, G. O.; Williams, P. A. (9 September 2011). Handbook of Food Proteins. Elsevier. ISBN 978-0-85709-363-9.
- ↑ Rubinsztein, David C.; Mariño, Guillermo; Kroemer, Guido (2 September 2011). "Autophagy and aging". Cell. 146 (5): 682–695. ISSN 1097-4172. doi:10.1016/j.cell.2011.07.030.
- ↑ Pasiakos, S. M.; McClung, H. L.; McClung, J. P.; Margolis, L. M.; Andersen, N. E.; Cloutier, G. J.; Pikosky, M. A.; Rood, J. C.; Fielding, R. A.; Young, A. J. (1 September 2011). "Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis". American Journal of Clinical Nutrition. 94 (3): 809–818. doi:10.3945/ajcn.111.017061.
- ↑ Bollwein, Julia; Diekmann, Rebecca; Kaiser, Matthias J.; Bauer, Jürgen M.; Uter, Wolfgang; Sieber, Cornel C.; Volkert, Dorothee (5 August 2013). "Distribution but not amount of protein intake is associated with frailty: a cross-sectional investigation in the region of Nürnberg". Nutrition Journal. 12: 109. ISSN 1475-2891. doi:10.1186/1475-2891-12-109.
- ↑ Schwingshackl, L., & Hoffmann, G. (2014). Comparison of high vs normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta-analysis. PLoS One 9(5): e97656.
- ↑ Paddon-Jones, Douglas; Rasmussen, Blake B. (January 2009). "Dietary protein recommendations and the prevention of sarcopenia". Current Opinion in Clinical Nutrition and Metabolic Care. 12 (1): 86–90. ISSN 1473-6519. doi:10.1097/MCO.0b013e32831cef8b.
- ↑ Rodriguez, Nancy R. (1 June 2014). "Protein-Centric Meals for Optimal Protein Utilization: Can It Be That Simple?". The Journal of Nutrition. 144 (6): 797–798. doi:10.3945/jn.114.193615.
- ↑ Layman, Donald K; Anthony, Tracy G; Rasmussen, Blake B; Adams, Sean H; Lynch, Christopher J; Brinkworth, Grant D; Davis, Teresa A (1 June 2015). "Defining meal requirements for protein to optimize metabolic roles of amino acids". The American Journal of Clinical Nutrition. 101 (6): 1330S–1338S. doi:10.3945/ajcn.114.084053.
- ↑ "A Closer Look at Current Intakes and Recommended Shifts - 2015-2020 Dietary Guidelines - health.gov". web.archive.org. 9 January 2016. Retrieved 4 October 2021.
- ↑ Vegetarian Diets American Heart Association, Sep 26, 2016
- ↑ Kalantar-Zadeh, Kamyar; Fouque, Denis (2 November 2017). "Nutritional Management of Chronic Kidney Disease". New England Journal of Medicine. 377 (18): 1765–1776. doi:10.1056/NEJMra1700312.
- ↑ Yu, Zhangping; Nan, Fengwei; Wang, Leslie Yingzhijie; Jiang, Hua; Chen, Wei; Jiang, Yu (June 2020). "Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials". Clinical Nutrition. 39 (6): 1724–1734. doi:10.1016/j.clnu.2019.08.008.